Exam 20: The Chemistry of Carboxylic Acids
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
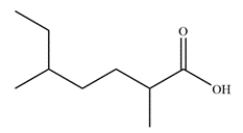
Name the carboxylic acid.
Free
(Essay)
4.9/5  (31)
(31)
Correct Answer:
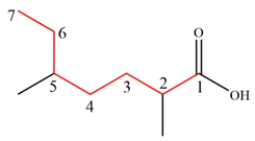
The longest chain containing the carboxylic acid is highlighted in red below. The name is 2,5-dimethylheptanoic acid.
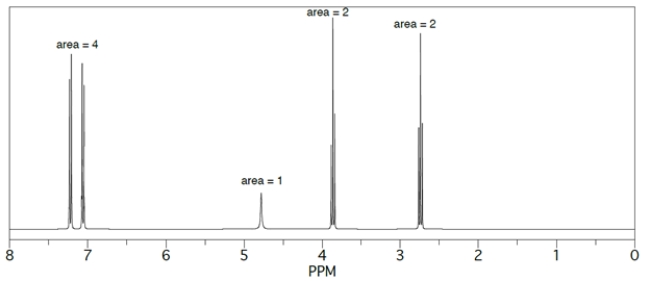
Provide structures for products A-D. The 1H NMR spectrum of D is given.
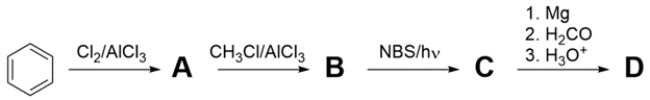
Free
(Essay)
4.8/5  (32)
(32)
Correct Answer:
Step 1 is chlorination of benzene. Step 2 is the Friedel-Crafts alkylation of chlorobenzene. Step 3 is the bromination of the benzyl protons to give a benzyl bromide. Step 4 converts the bromide to a Grignard reagent, then reacts with formaldehyde to give a primary alcohol.
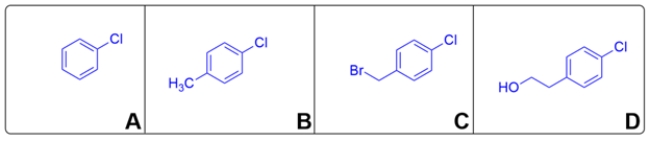 Compound D matches the 1H NMR spectra. The four protons >7ppm belong to the aromatic protons. The singlet is the hydroxy proton. The two triplets belong to the -CH2CH2- group.
Compound D matches the 1H NMR spectra. The four protons >7ppm belong to the aromatic protons. The singlet is the hydroxy proton. The two triplets belong to the -CH2CH2- group.
Which of the carboxylic acids would not decarboxylate (lose CO2) upon warming?
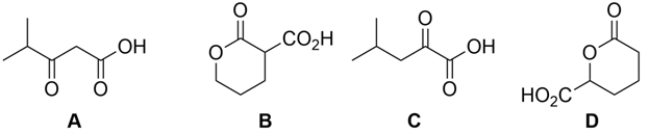
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
E
Draw the structure of the nucleophile (including formal charges) that would give the product on the right.
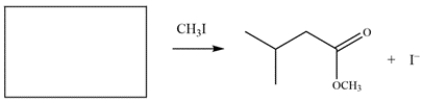
(Essay)
4.8/5  (32)
(32)
Draw the structure of the nucleophile (including formal charges) that would give the product on the right.
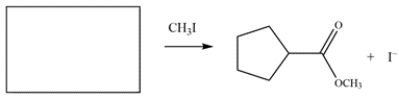
(Essay)
4.8/5  (32)
(32)
Predict the major organic product for the reaction. If you believe no reaction would occur, write NR.
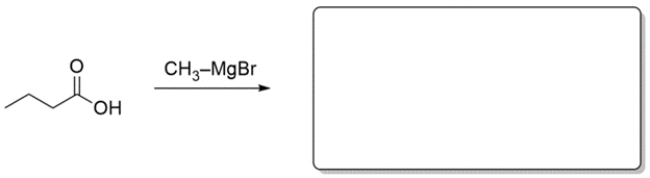
(Essay)
4.7/5  (33)
(33)
A student has synthesized two isomers, A and B, but neglected to label the flasks. Suggest a spectroscopy method the student could use to quickly differentiate between them and explain what feature they would be looking for.
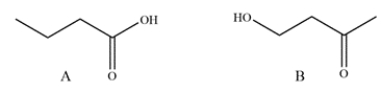
(Essay)
4.9/5  (34)
(34)
Rank the functional groups in order of highest priority to lowest for nomenclature.
(Multiple Choice)
4.7/5  (40)
(40)
Outline a multistep synthesis of the ketone from the carboxylic acid.
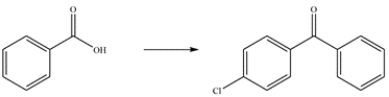
(Essay)
4.8/5  (34)
(34)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)