Deck 19: The Chemistry of Aldehydes and Ketones
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 19: The Chemistry of Aldehydes and Ketones
1
Which of the reactions would have the largest Keq?
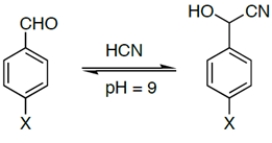
A) X = H
B) X = OEt
C) X = CN
D) X = NO2
E) X = Br

A) X = H
B) X = OEt
C) X = CN
D) X = NO2
E) X = Br
D
2
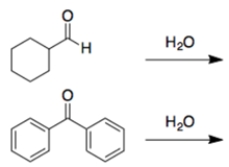
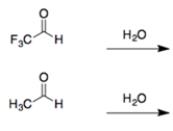
In each set of reactions, circle the one with the larger equilibrium constant (lies further to the right).
a
.
 b.
b.
a
.
 b.
b. 
a.
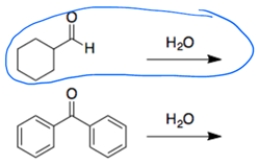 In both cases, a carbonyl is treated with water to form a hydrate. In general, addition is more favorable for aldehydes than ketones. The second reaction is less favorable since the benzene rings can donate electrons by resonance to the carbonyl carbon.
In both cases, a carbonyl is treated with water to form a hydrate. In general, addition is more favorable for aldehydes than ketones. The second reaction is less favorable since the benzene rings can donate electrons by resonance to the carbonyl carbon.
b.
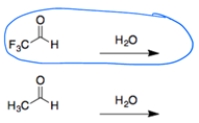 In both cases, an aldehyde reacts with water to form a hydrate. The aldehyde with the electron-withdrawing fluorines will make the carbonyl addition more favorable due to the polar effect.
In both cases, an aldehyde reacts with water to form a hydrate. The aldehyde with the electron-withdrawing fluorines will make the carbonyl addition more favorable due to the polar effect.
 In both cases, a carbonyl is treated with water to form a hydrate. In general, addition is more favorable for aldehydes than ketones. The second reaction is less favorable since the benzene rings can donate electrons by resonance to the carbonyl carbon.
In both cases, a carbonyl is treated with water to form a hydrate. In general, addition is more favorable for aldehydes than ketones. The second reaction is less favorable since the benzene rings can donate electrons by resonance to the carbonyl carbon.
b.
 In both cases, an aldehyde reacts with water to form a hydrate. The aldehyde with the electron-withdrawing fluorines will make the carbonyl addition more favorable due to the polar effect.
In both cases, an aldehyde reacts with water to form a hydrate. The aldehyde with the electron-withdrawing fluorines will make the carbonyl addition more favorable due to the polar effect. 3
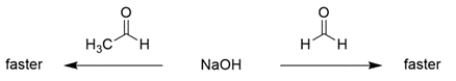
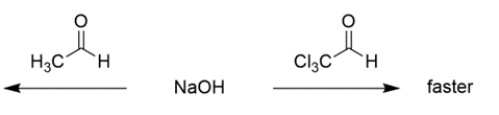
For each pair of reactions, circle "faster" for the one you believe is faster.
a.
b.
a.

b.

a. The unsubstituted aldehyde will react faster, since alkyl groups stabilize carbocations.
b. The aldehyde with chlorines will react faster, since electronegative groups near the carbonyl make carbonyl addition more favorable due to the polar effect.
b. The aldehyde with chlorines will react faster, since electronegative groups near the carbonyl make carbonyl addition more favorable due to the polar effect.
4
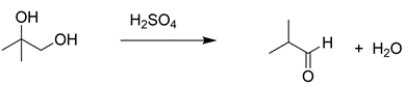
Provide a detailed, arrow-pushing mechanism for the transformation. Show all reactive intermediates. (It is not necessary to show every resonance structure for appropriate intermediates.)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
-Methoxyglucose contains which functional group?
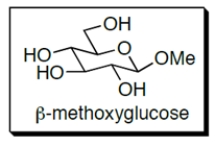
A) a hemiacetal
B) an acetal
C) an imine
D) an ester
E) more than one of these

A) a hemiacetal
B) an acetal
C) an imine
D) an ester
E) more than one of these

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
Predict the major organic product of the reaction. If you believe no reaction would occur, write NR and explain why.
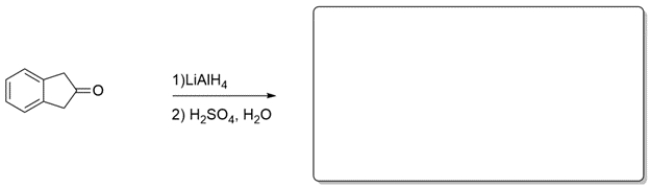


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the missing reagent for the reaction.
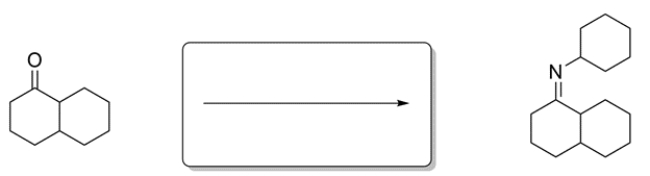


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the missing reagent for the reaction.
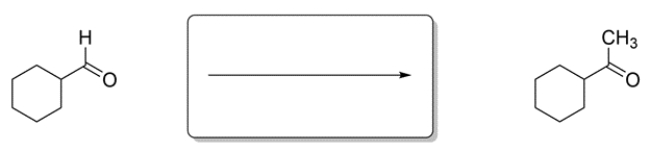


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Outline a synthesis to achieve this transformation.
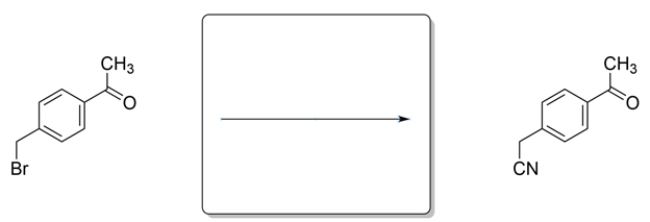


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Two students working in a laboratory are tasked to synthesize compound B from compound A. The first student thinks that a dehydration reaction, treating A with strong acid and heat, will do the trick. The second student disagrees and says the reaction will not work as intended.
a. Explain why the second student is right and predict the more likely product (C) of the reaction.
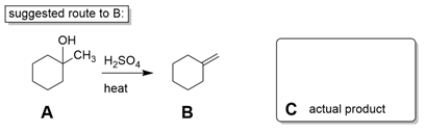
b. If you could start with cyclohexanone, how would you prepare B? You may use any other reagents you want. Show all steps and intermediates, but reaction mechanisms are not necessary.
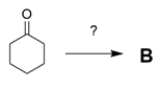
a. Explain why the second student is right and predict the more likely product (C) of the reaction.

b. If you could start with cyclohexanone, how would you prepare B? You may use any other reagents you want. Show all steps and intermediates, but reaction mechanisms are not necessary.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
Provide detailed, arrow-pushing mechanisms for the two-step transformation. Show all reactive intermediates, and all proton transfer steps. (It is not necessary to show every resonance structure for intermediates.)
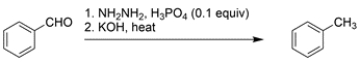


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Deduce the structure of the missing starting material.
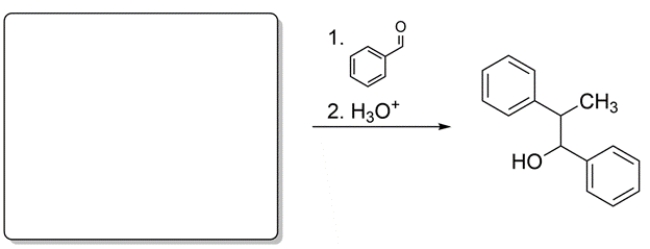


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Bases can catalyze nucleophilic addition reactions of aldehydes and ketones by
A) shifting the equilibrium of the reaction.
B) converting water to hydroxide ion, a much better nucleophile.
C) making the carbonyl group more electrophilic.
D) making the carbonyl group less electrophilic.
A) shifting the equilibrium of the reaction.
B) converting water to hydroxide ion, a much better nucleophile.
C) making the carbonyl group more electrophilic.
D) making the carbonyl group less electrophilic.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Predict the major organic product for the reaction in the box provided. If you believe no reaction would occur, write NR.
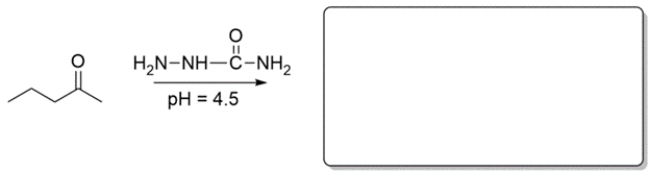


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Outline a synthesis for the transformation. Identify the missing reagents.



Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
The rate of formation of the oxime functional group from acetone and hydroxylamine in water is pH dependent. The ideal pH for this reaction is about 4.8.
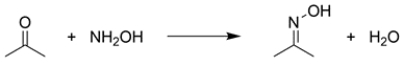 a. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 1.
a. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 1.
b. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 8.
 a. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 1.
a. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 1.b. With reference to the reaction mechanism, using structures and 15 words or less, explain why this reaction is very slow at pH = 8.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
The conversion of alkyl azides into primary amines through a procedure called the Staudinger reaction is shown.
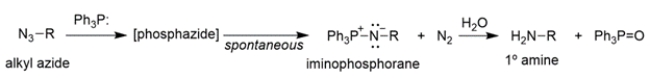 The mechanism of the first part of the reaction is conceptually similar to the Wittig reaction that we've talked about, where an ylide reacts with a carbonyl to yield an oxaphosphetane, which then rearranges to an alkene and triphenylphosphine oxide.
The mechanism of the first part of the reaction is conceptually similar to the Wittig reaction that we've talked about, where an ylide reacts with a carbonyl to yield an oxaphosphetane, which then rearranges to an alkene and triphenylphosphine oxide.
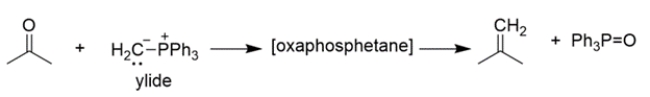 Provide an arrow pushing mechanism for the first part of the Staudinger reaction, the reaction of triphenylphosphine (TPP) and an alkyl azide to produce an iminophosphorane and nitrogen gas.
Provide an arrow pushing mechanism for the first part of the Staudinger reaction, the reaction of triphenylphosphine (TPP) and an alkyl azide to produce an iminophosphorane and nitrogen gas.
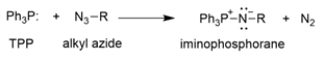
 The mechanism of the first part of the reaction is conceptually similar to the Wittig reaction that we've talked about, where an ylide reacts with a carbonyl to yield an oxaphosphetane, which then rearranges to an alkene and triphenylphosphine oxide.
The mechanism of the first part of the reaction is conceptually similar to the Wittig reaction that we've talked about, where an ylide reacts with a carbonyl to yield an oxaphosphetane, which then rearranges to an alkene and triphenylphosphine oxide.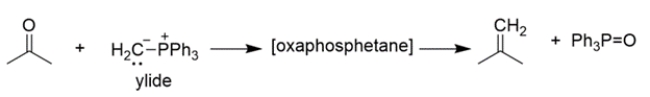 Provide an arrow pushing mechanism for the first part of the Staudinger reaction, the reaction of triphenylphosphine (TPP) and an alkyl azide to produce an iminophosphorane and nitrogen gas.
Provide an arrow pushing mechanism for the first part of the Staudinger reaction, the reaction of triphenylphosphine (TPP) and an alkyl azide to produce an iminophosphorane and nitrogen gas.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Predict the major organic product of the reaction, for which the proton NMR spectrum is provided.
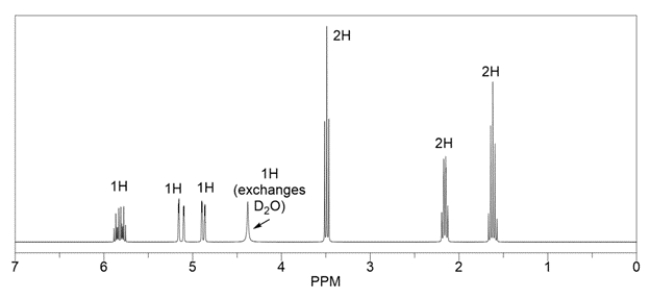



Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Predict the major organic product in the box provided. There may be more than one acceptable answer. If you believe no reaction would occur, write NR. Denote stereochemistry where appropriate.
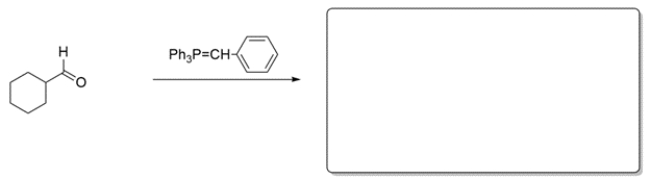


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
Treatment of a mixture of cyclopentanone (A) and acetone cyanohydrin (B) with a catalytic amount of base (NaOH) leads to an equilibration to form a mixture of A, B, cyclopentanone cyanohydrin (C), and acetone (D). Provide an arrow pushing mechanism for the transformation in the forward direction (A + B → C + D) as written.
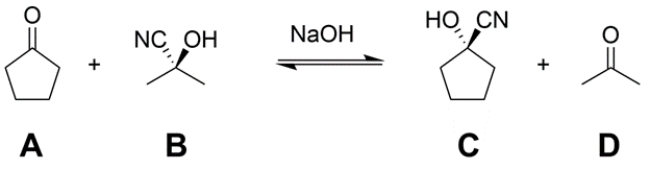


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
In the reaction of H2O at pH 2 with these compounds, the equilibrium would most favor the geminal dihydroxy product or hydrate for which starting material?
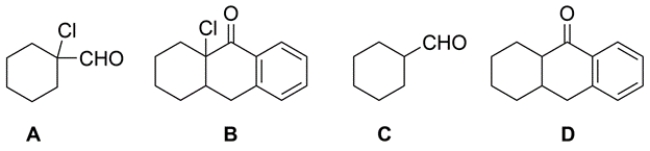
A) compound A
B) compound B
C) compound C
D) compound D
E) can't tell without knowing the corresponding trend for the rates of the reactions

A) compound A
B) compound B
C) compound C
D) compound D
E) can't tell without knowing the corresponding trend for the rates of the reactions

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Draw the structure of the major organic product for the reaction. If no reaction would occur, write NR. Unless otherwise stated assume there is enough of any reagent to do all the normal reactions. Reaction mechanisms are not necessary.
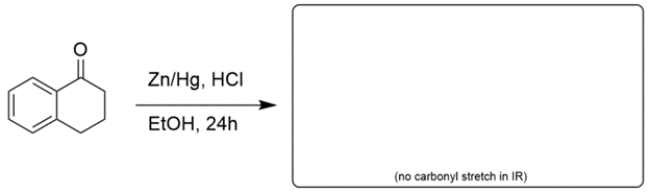


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Shown are two acetals. In the corresponding box, draw the structures of the carbonyl compound and alcohol component pieces for each acetal.
a.
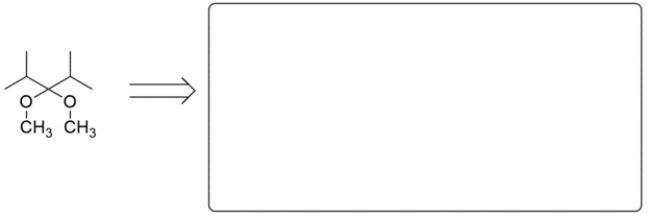
b.
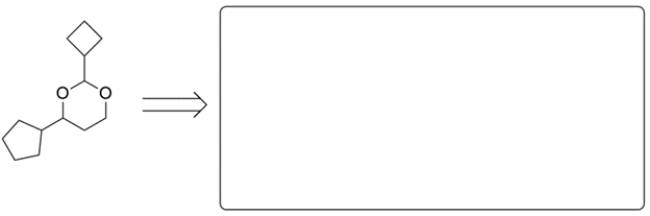
a.

b.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
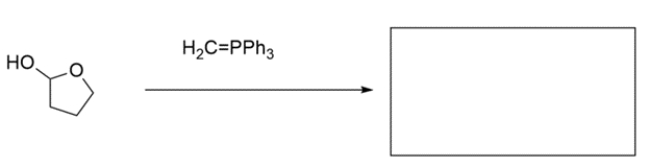
These two alkenes were prepared by Wittig reactions. In the corresponding box show one set of the carbonyl and ylide reaction partners that would make each alkene. There may be more than one correct answer; just show one. Remember the general form for an ylide is Ph3P=CR2.
a.
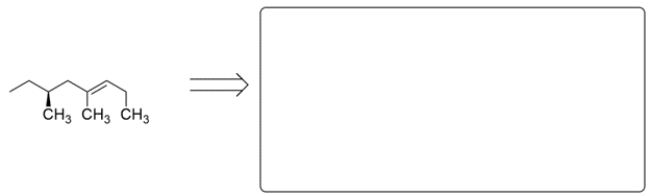
b.
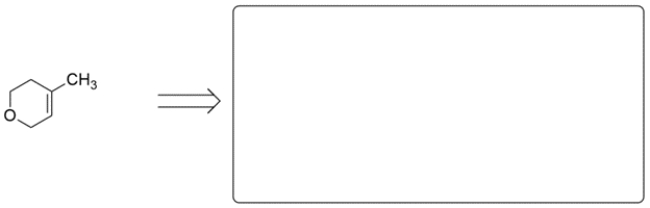
a.

b.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Outline a synthetic route for the transformation:
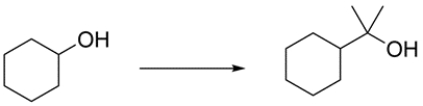


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


