Exam 19: The Chemistry of Aldehydes and Ketones
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
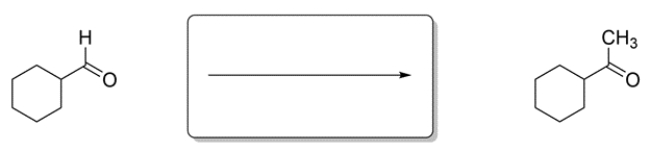
Identify the missing reagent for the reaction.
Free
(Essay)
4.9/5  (32)
(32)
Correct Answer:
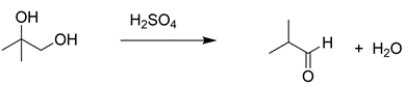
 The overall reaction is a substitution of an aldehyde hydrogen with a methyl group. There is no direct way of achieving this transformation, so consider the methods you know for generating a carbon-carbon bond. A Grignard addition to the aldehyde would generate a secondary alcohol. The alcohol could then be oxidized to the ketone.
The overall reaction is a substitution of an aldehyde hydrogen with a methyl group. There is no direct way of achieving this transformation, so consider the methods you know for generating a carbon-carbon bond. A Grignard addition to the aldehyde would generate a secondary alcohol. The alcohol could then be oxidized to the ketone.
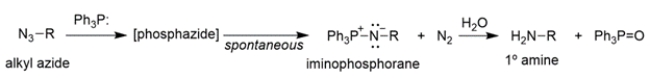
The conversion of alkyl azides into primary amines through a procedure called the Staudinger reaction is shown.
 The mechanism of the first part of the reaction is conceptually similar to the Wittig reaction that we've talked about, where an ylide reacts with a carbonyl to yield an oxaphosphetane, which then rearranges to an alkene and triphenylphosphine oxide.
The mechanism of the first part of the reaction is conceptually similar to the Wittig reaction that we've talked about, where an ylide reacts with a carbonyl to yield an oxaphosphetane, which then rearranges to an alkene and triphenylphosphine oxide.
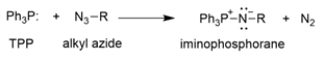
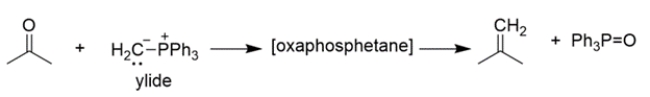 Provide an arrow pushing mechanism for the first part of the Staudinger reaction, the reaction of triphenylphosphine (TPP) and an alkyl azide to produce an iminophosphorane and nitrogen gas.
Provide an arrow pushing mechanism for the first part of the Staudinger reaction, the reaction of triphenylphosphine (TPP) and an alkyl azide to produce an iminophosphorane and nitrogen gas.
Free
(Essay)
4.9/5  (32)
(32)
Correct Answer:
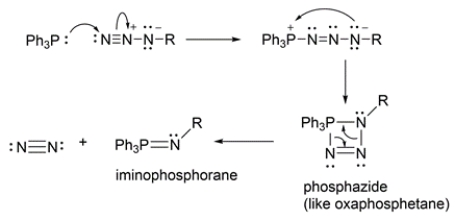
Provide a detailed, arrow-pushing mechanism for the transformation. Show all reactive intermediates. (It is not necessary to show every resonance structure for appropriate intermediates.)
Free
(Essay)
4.8/5  (37)
(37)
Correct Answer:
This reaction is known as the pinacol rearrangement, where a 1,2-diol under acidic conditions will rearrange to give a carbonyl. The mechanism involves:
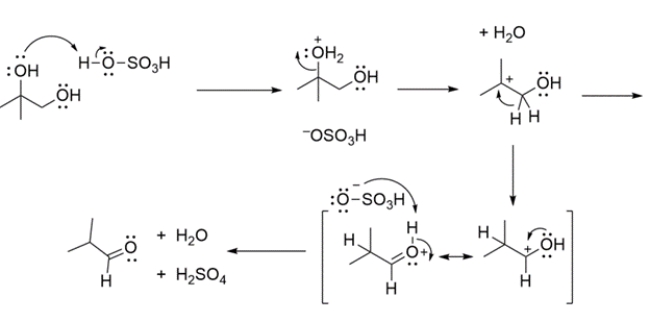
Predict the major organic product in the box provided. There may be more than one acceptable answer. If you believe no reaction would occur, write NR. Denote stereochemistry where appropriate.
(Essay)
4.7/5  (31)
(31)
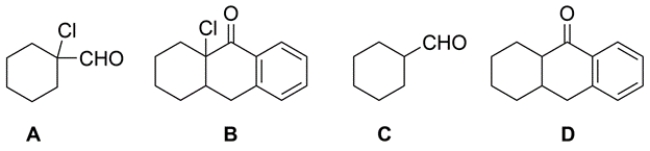
Two students working in a laboratory are tasked to synthesize compound B from compound A. The first student thinks that a dehydration reaction, treating A with strong acid and heat, will do the trick. The second student disagrees and says the reaction will not work as intended.
a. Explain why the second student is right and predict the more likely product (C) of the reaction.
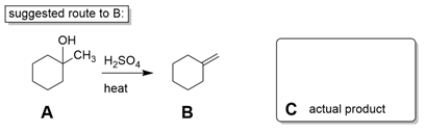
b. If you could start with cyclohexanone, how would you prepare B? You may use any other reagents you want. Show all steps and intermediates, but reaction mechanisms are not necessary.
b. If you could start with cyclohexanone, how would you prepare B? You may use any other reagents you want. Show all steps and intermediates, but reaction mechanisms are not necessary.
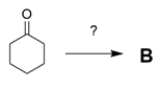
(Essay)
4.8/5  (36)
(36)
For each pair of reactions, circle "faster" for the one you believe is faster.
a.
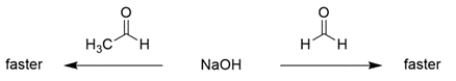
b.
b.
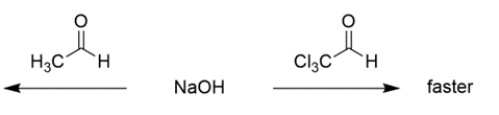
(Essay)
4.9/5  (34)
(34)
Predict the major organic product of the reaction. If you believe no reaction would occur, write NR and explain why.
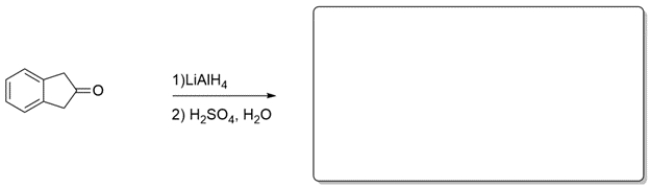
(Essay)
4.7/5  (37)
(37)
Treatment of a mixture of cyclopentanone (A) and acetone cyanohydrin (B) with a catalytic amount of base (NaOH) leads to an equilibration to form a mixture of A, B, cyclopentanone cyanohydrin (C), and acetone (D). Provide an arrow pushing mechanism for the transformation in the forward direction (A + B → C + D) as written.
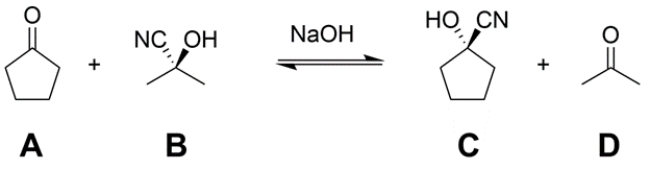
(Essay)
4.7/5  (40)
(40)
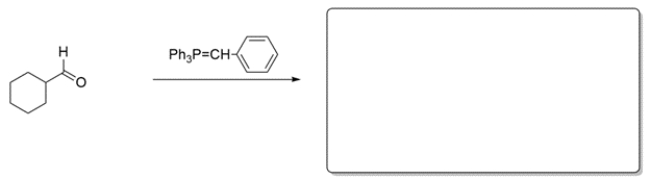
These two alkenes were prepared by Wittig reactions. In the corresponding box show one set of the carbonyl and ylide reaction partners that would make each alkene. There may be more than one correct answer; just show one. Remember the general form for an ylide is Ph3P=CR2.
a.
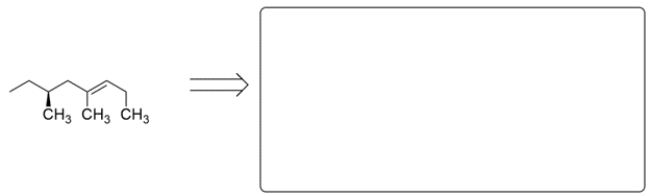
b.
b.
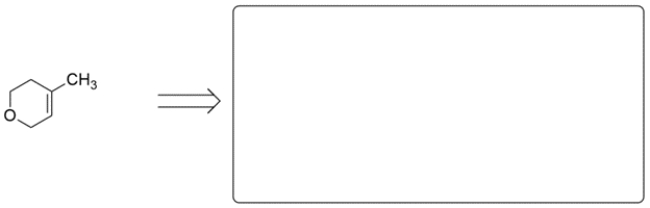
(Essay)
4.8/5  (39)
(39)
Provide detailed, arrow-pushing mechanisms for the two-step transformation. Show all reactive intermediates, and all proton transfer steps. (It is not necessary to show every resonance structure for intermediates.)
(Essay)
4.8/5  (31)
(31)
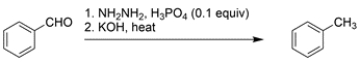
Outline a synthesis for the transformation. Identify the missing reagents.

(Essay)
4.9/5  (41)
(41)
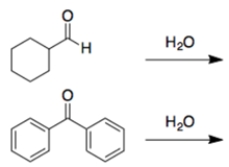
In the reaction of H2O at pH 2 with these compounds, the equilibrium would most favor the geminal dihydroxy product or hydrate for which starting material?
(Multiple Choice)
4.8/5  (39)
(39)
In each set of reactions, circle the one with the larger equilibrium constant (lies further to the right).
a
.
 b.
b.
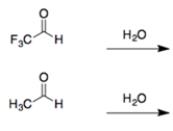
(Essay)
4.9/5  (30)
(30)
Draw the structure of the major organic product for the reaction. If no reaction would occur, write NR. Unless otherwise stated assume there is enough of any reagent to do all the normal reactions. Reaction mechanisms are not necessary.
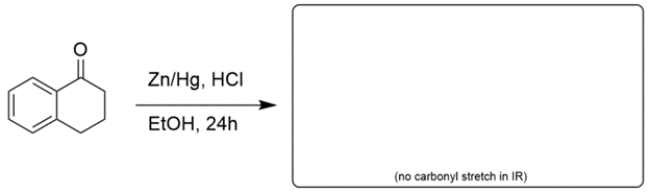
(Essay)
4.8/5  (37)
(37)
Predict the major organic product for the reaction in the box provided. If you believe no reaction would occur, write NR.
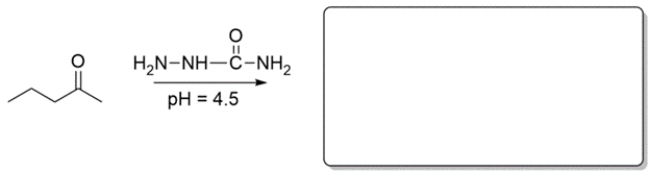
(Essay)
4.7/5  (32)
(32)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)