Deck 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols
1
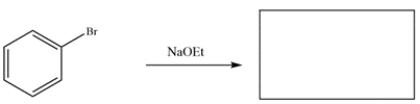
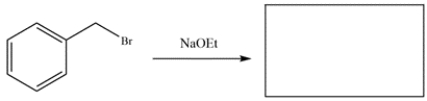
Predict the major organic products of the reactions. If no reaction, write NR and explain why no reaction occurs. Compare the two reactions and identify which will occur faster.
a.
 b.
b.
a.
 b.
b. 
a. Bromobenzene will not react with NaOEt. Recall that vinyl halides are unreactive in either SN1 or SN2 reactions. Aryl halides can undergo nucleophilic aromatic substitution, but the benzene ring often requires an electron withdrawing group in the ortho or para position to drive the reaction.
b. The starting material is benzyl bromide, so it will readily undergo SN2 substitution with ethoxide to form an ether.
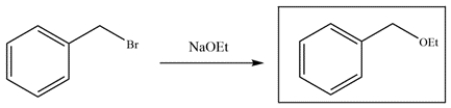
Since the benzyl halide is more reactive than the aryl halide, reaction b is faster.
b. The starting material is benzyl bromide, so it will readily undergo SN2 substitution with ethoxide to form an ether.

Since the benzyl halide is more reactive than the aryl halide, reaction b is faster.
2
Analyze the three compounds and identify which would react fastest with NaI and which would react slowest. Explain your reasoning.
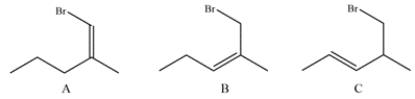

Compound A is a vinyl halide and will be unreactive with nucleophiles in the SN2 reaction. Compound B is an allylic primary bromide and compound C is a primary alkyl bromide. An allylic halide will be more reactive than its analogous non-alyllic counterpart, so compound B will be the fastest. Compound A will be the slowest.
3
Identify the most acidic compound.
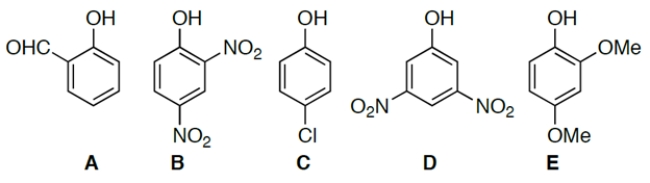
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

A) compound A
B) compound B
C) compound C
D) compound D
E) compound E
B
4
Arrange the compounds in order of increasing acidity (least to most acidic.)
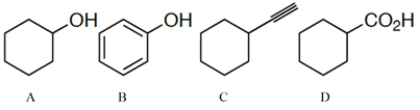
A) A < C < D < B
B) A < D < C < B
C) A < B < C < D
D) C < A < B < D
E) C < A < D < B

A) A < C < D < B
B) A < D < C < B
C) A < B < C < D
D) C < A < B < D
E) C < A < D < B

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
In each set of reactions, circle the one with the larger equilibrium constant (lies further to the right).
a.
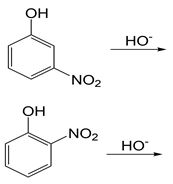
b.
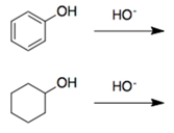
a.

b.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
In each set of reactions, circle the one that occurs faster.
a.
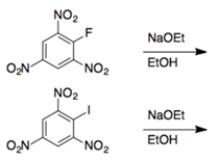
b.
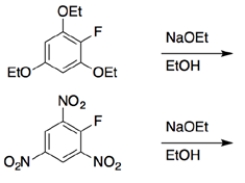
a.

b.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
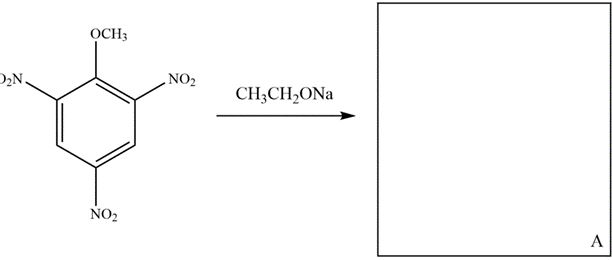
7
Reaction of 2,4,6-trinitroanisole with sodium ethoxide forms a stable anion, A. Draw the structure of A in the box, depicting the most stable resonance contributor (not hybrid) and provide an arrow pushing mechanism for its formation.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
1. Fluoxetine, also known as Prozac, is prepared from intermediate compound A. Intermediate A can be prepared by a reaction between reagents B and C. Using the spectral data provided in the boxes, provide structures for B and C.
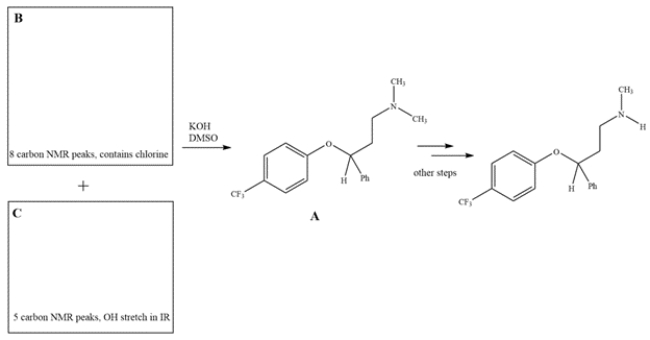 2. The physiologically active form of fluoxetine has (S) stereochemistry. Clearly draw wedge-and-dash bonds to your compound B, showing the stereochemistry needed to give S-fluoxetine.
2. The physiologically active form of fluoxetine has (S) stereochemistry. Clearly draw wedge-and-dash bonds to your compound B, showing the stereochemistry needed to give S-fluoxetine.
3. The 1H NMR spectrum of B or C is shown below. Match the spectra with either B or C and label in the box.
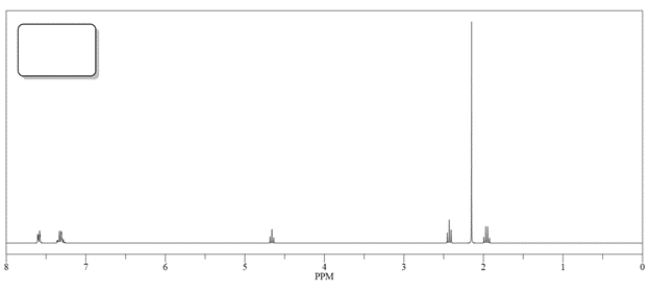
 2. The physiologically active form of fluoxetine has (S) stereochemistry. Clearly draw wedge-and-dash bonds to your compound B, showing the stereochemistry needed to give S-fluoxetine.
2. The physiologically active form of fluoxetine has (S) stereochemistry. Clearly draw wedge-and-dash bonds to your compound B, showing the stereochemistry needed to give S-fluoxetine.3. The 1H NMR spectrum of B or C is shown below. Match the spectra with either B or C and label in the box.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Provide detailed, arrow-pushing mechanisms for the transformation. Show all reactive intermediates and proton transfer steps. (It is not necessary to show every resonance structure for intermediates.)
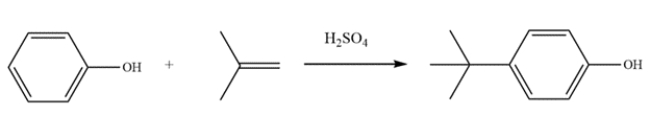


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Outline a synthesis of this alkyne, using the given starting material and any other reagents you need.
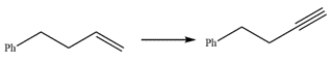


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
The alkyne can be formed from 1,1-dibromo-3-methylbutane. Draw a curved arrow mechanism explaining this transformation.
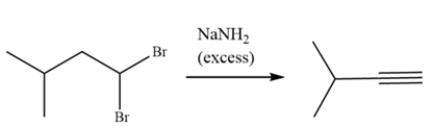


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Outline a four-step synthesis to transform 1-butene to (E)-3-hexene.
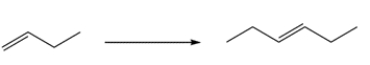


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Outline a multistep synthesis for the compound from chlorobenzene.
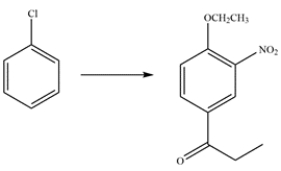


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Octyl-4-methoxycinnamate is an ingredient found in sunscreens to block UV-B rays. Deduce the starting materials that could form octyl-4-methoxycinnamate from the Heck reaction.
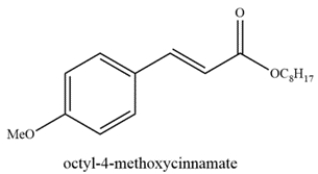


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Ginkgo Biloba is a supplement sold over the counter proposed to help memory and act as an antioxidant. Ginkgo Biloba leaves contain alkylphenols, such as ginkgolic acid. A key intermediate in the synthesis of ginkgolic acid is formed from a Heck reaction with an aryl triflate (OTf) instead of aryl halide. Given the structure of the aryl triflate and alkene, predict the structure of the intermediate.
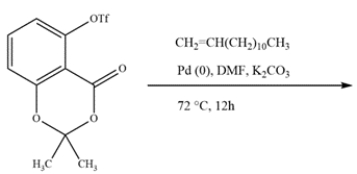


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
Predict the major organic product for the reaction:


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
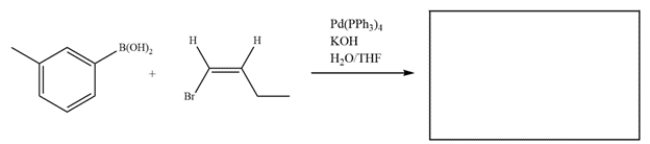
Deduce the starting materials that would give this product after a Suzuki coupling.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
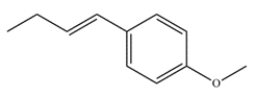
Predict the major organic product formed after alkene metathesis in the presence of an appropriate ruthenium catalyst. The 13C NMR data of the product show 4 peaks, at 40, 57, 77, and 128 ppm.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
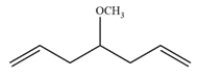
Outline an alkene metathesis reaction that would give this compound as the major product.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
Ubiquinone, also known as coenzyme Q, is a biological quinone found to be involved in electron transport. A student performs a reduction of ubiquinone and monitors the reaction by IR spectroscopy. The student observes a decrease in the peak at 1650 cm‒1 and the appearance of a large broad stretch at 3300 cm‒1. Deduce the structure of the reduced product.
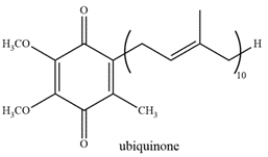


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
Which reagent is used in the Stille coupling?
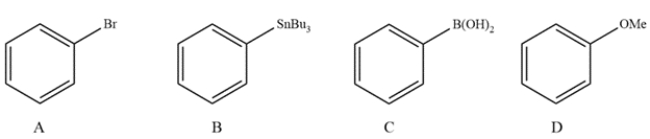
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Predict the major organic product for the reaction.
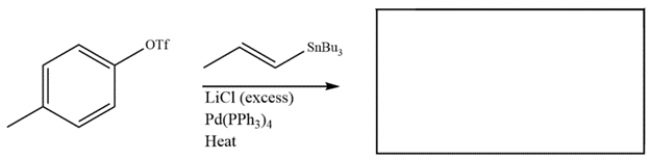


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Palladium tetrakis is shown.
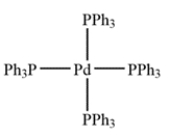 a. Identify the oxidation state of the palladium. ________________
a. Identify the oxidation state of the palladium. ________________
b. Identify the dn count (that is, the value of n): ________________ electrons.
c. The total electron count around the metal is ________________ electrons.
 a. Identify the oxidation state of the palladium. ________________
a. Identify the oxidation state of the palladium. ________________b. Identify the dn count (that is, the value of n): ________________ electrons.
c. The total electron count around the metal is ________________ electrons.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the transition metal complex [Mn(CN)6]3-.
a. Identify the oxidation state of the Mn. ________________
b. Identify the dn count (that is, the value of n): ________________ electrons.
c. The total electron count around the metal is ________________ electrons.
a. Identify the oxidation state of the Mn. ________________
b. Identify the dn count (that is, the value of n): ________________ electrons.
c. The total electron count around the metal is ________________ electrons.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the transition-metal catalyzed reaction:
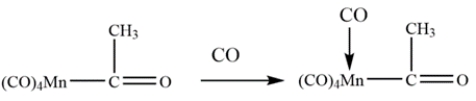
A) ligand association
B) ligand dissociation
C) ligand insertion
D) oxidative addition

A) ligand association
B) ligand dissociation
C) ligand insertion
D) oxidative addition

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


