Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols
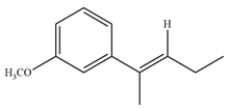
Predict the major organic product formed after alkene metathesis in the presence of an appropriate ruthenium catalyst. The 13C NMR data of the product show 4 peaks, at 40, 57, 77, and 128 ppm.
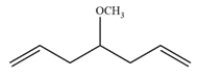
In an alkene metathesis reaction, two alkenes are split and rearranged. In this starting material, both alkenes are part of the same molecule, so a ring closing metathesis occurs. The by-product is ethylene gas, which drives the reaction to completion.
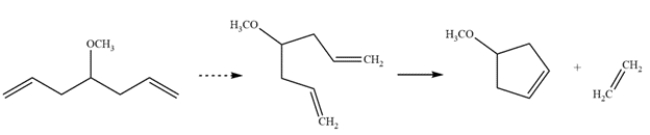 The resulting product is a cyclopentene ring with a methoxy substituent. Let's check that this matches the 13C NMR data. The molecule is symmetric, with a plane of symmetry bisecting the alkene and methoxy group. The two alkene carbons are represented by the peak at 128 ppm. The remaining ring carbons are represented by the peak at 40 ppm. The carbons directly bonded to the oxygen are represented by the peaks at 57 and 77 ppm.
The resulting product is a cyclopentene ring with a methoxy substituent. Let's check that this matches the 13C NMR data. The molecule is symmetric, with a plane of symmetry bisecting the alkene and methoxy group. The two alkene carbons are represented by the peak at 128 ppm. The remaining ring carbons are represented by the peak at 40 ppm. The carbons directly bonded to the oxygen are represented by the peaks at 57 and 77 ppm.
Provide detailed, arrow-pushing mechanisms for the transformation. Show all reactive intermediates and proton transfer steps. (It is not necessary to show every resonance structure for intermediates.)
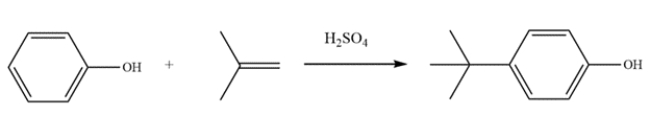
This reaction is the electrophilic aromatic substitution of a phenol. The first step is to generate the electrophile.
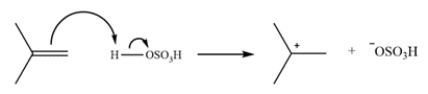 The second step is an electrophilic aromatic substitution of phenol. The sigma complex forms a carbocation that can be stabilized by the ring and the phenol oxygen.
The second step is an electrophilic aromatic substitution of phenol. The sigma complex forms a carbocation that can be stabilized by the ring and the phenol oxygen.
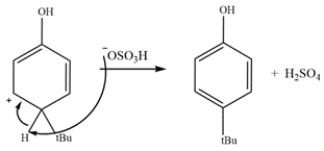
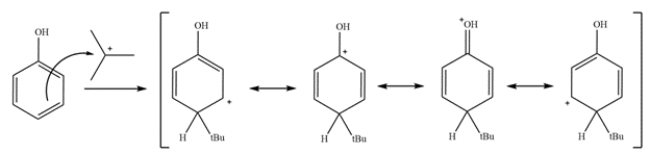 The last step is deprotonation and re-aromatization to give the products.
The last step is deprotonation and re-aromatization to give the products.
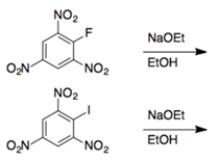
In each set of reactions, circle the one that occurs faster.
a.
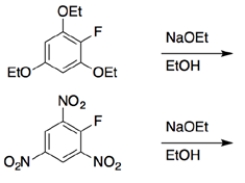
b.
b.
a. These reactions will be a nucleophilic aromatic substitution, where the halide is substituted by ethoxide. An aryl fluoride is much more reactive than an aryl iodide in a nucleophilic aromatic substitution, so the reaction containing the fluoride will be faster.
b. These reactions will be a nucleophilic aromatic substitution, where the halide is substituted by ethoxide. An aryl fluoride that has substituents that can stabilize the anion formed after nucleophilic addition will be faster. The nitro groups on the ortho and para positions can accept the anion, thus the nitro containing aryl fluoride will be faster.
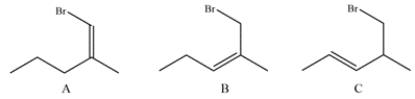
Analyze the three compounds and identify which would react fastest with NaI and which would react slowest. Explain your reasoning.
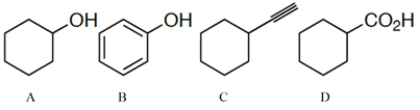
Arrange the compounds in order of increasing acidity (least to most acidic.)
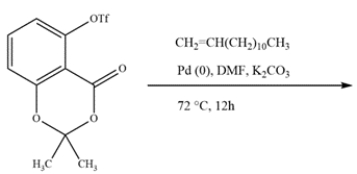
Ginkgo Biloba is a supplement sold over the counter proposed to help memory and act as an antioxidant. Ginkgo Biloba leaves contain alkylphenols, such as ginkgolic acid. A key intermediate in the synthesis of ginkgolic acid is formed from a Heck reaction with an aryl triflate (OTf) instead of aryl halide. Given the structure of the aryl triflate and alkene, predict the structure of the intermediate.
1. Fluoxetine, also known as Prozac, is prepared from intermediate compound A. Intermediate A can be prepared by a reaction between reagents B and C. Using the spectral data provided in the boxes, provide structures for B and C.
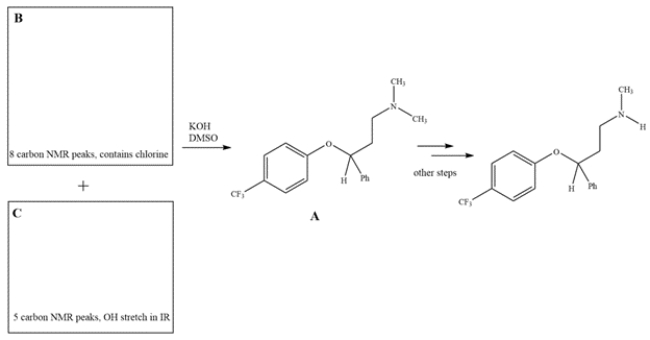 2. The physiologically active form of fluoxetine has (S) stereochemistry. Clearly draw wedge-and-dash bonds to your compound B, showing the stereochemistry needed to give S-fluoxetine.
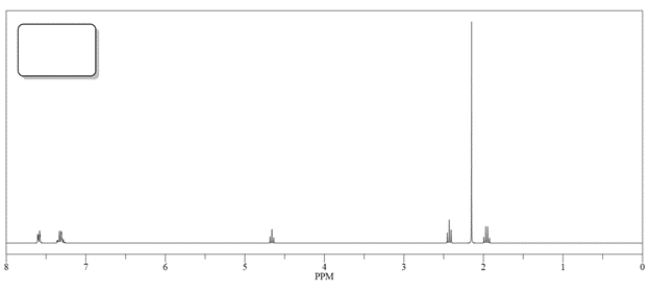
3. The 1H NMR spectrum of B or C is shown below. Match the spectra with either B or C and label in the box.
2. The physiologically active form of fluoxetine has (S) stereochemistry. Clearly draw wedge-and-dash bonds to your compound B, showing the stereochemistry needed to give S-fluoxetine.
3. The 1H NMR spectrum of B or C is shown below. Match the spectra with either B or C and label in the box.
Consider the transition metal complex [Mn(CN)6]3-.
a. Identify the oxidation state of the Mn. ________________
b. Identify the dn count (that is, the value of n): ________________ electrons.
c. The total electron count around the metal is ________________ electrons.
Predict the major organic products of the reactions. If no reaction, write NR and explain why no reaction occurs. Compare the two reactions and identify which will occur faster.
a.
 b.
b.
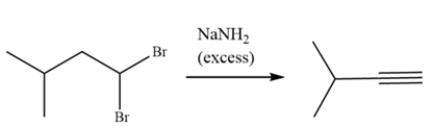
The alkyne can be formed from 1,1-dibromo-3-methylbutane. Draw a curved arrow mechanism explaining this transformation.
Palladium tetrakis is shown.
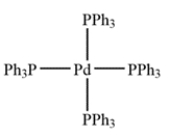 a. Identify the oxidation state of the palladium. ________________
b. Identify the dn count (that is, the value of n): ________________ electrons.
c. The total electron count around the metal is ________________ electrons.
a. Identify the oxidation state of the palladium. ________________
b. Identify the dn count (that is, the value of n): ________________ electrons.
c. The total electron count around the metal is ________________ electrons.
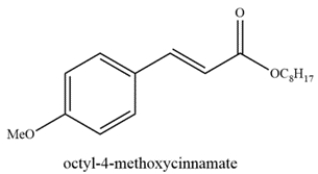
Octyl-4-methoxycinnamate is an ingredient found in sunscreens to block UV-B rays. Deduce the starting materials that could form octyl-4-methoxycinnamate from the Heck reaction.
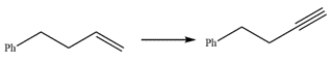
Outline a synthesis of this alkyne, using the given starting material and any other reagents you need.
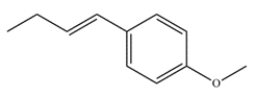
Deduce the starting materials that would give this product after a Suzuki coupling.
Outline an alkene metathesis reaction that would give this compound as the major product.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)