Deck 16: The Chemistry of Benzene and Its Derivatives
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/24
Play
Full screen (f)
Deck 16: The Chemistry of Benzene and Its Derivatives
1
Give the IUPAC name for the compound.
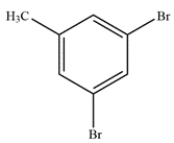

The compound is named 1,3-dibromo-5-methylbenzene. Alternatively, the parent name could include the methyl group in the benzene ring. The alternative name would be 3,5-dibromotoluene.
2
Give the IUPAC name for the compound.
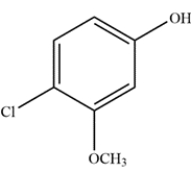

The benzene ring has a hydroxy substituent, so will have the parent name phenol. The hydroxy will have the highest priority, so it will receive the lowest locant numbering. Thus, the methoxy will be on carbon 3 and the chloro group on carbon 4. The complete name is thus 4-chloro-3-methoxyphenol.
3
Select the correct name for PhCH2Br.
A) bromobenzene
B) bromobenzyl
C) phenyl bromide
D) benzyl bromide
A) bromobenzene
B) bromobenzyl
C) phenyl bromide
D) benzyl bromide
D
4
A reaction forms the ortho, meta, and para isomers of dibromobenzene. Draw the structures of the three compounds and explain how you can use the melting point to separate the mixtures. Which would you expect to have the highest melting point?

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these statements is not true about the electrophilic bromination of benzene?
A) The reaction intermediate has an sp3 hybridized carbon.
B) A carbocation intermediate is formed in the rate limiting (slow) step of the reaction.
C) Aromaticity is regained when a proton is removed in the final step of the reaction.
D) Benzene functions as a nucleophile in the reaction.
E) A frequent by-product of the reaction is the normal bromine addition product seen with alkenes.
A) The reaction intermediate has an sp3 hybridized carbon.
B) A carbocation intermediate is formed in the rate limiting (slow) step of the reaction.
C) Aromaticity is regained when a proton is removed in the final step of the reaction.
D) Benzene functions as a nucleophile in the reaction.
E) A frequent by-product of the reaction is the normal bromine addition product seen with alkenes.

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
6
Which compound reacts slowest during nitration?
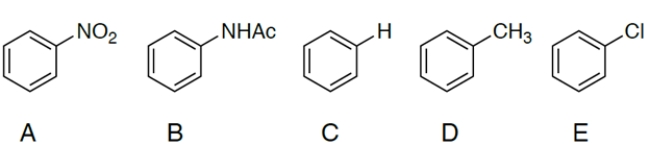
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these is a meta-directing group that is also an activator?
A) -CHO
B) -I
C) -NMe3+
D) -NO2
E) there are no meta-directing activators
A) -CHO
B) -I
C) -NMe3+
D) -NO2
E) there are no meta-directing activators

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
8
Which compound would be the most reactive towards ring bromination?
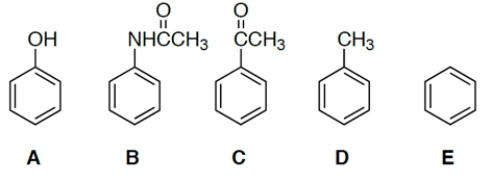
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
9
Indicate the missing products of the reaction. When filling in the product indicate only the major organic product(s). There may be more than one acceptable answer. If you believe no reaction would occur, write NR. Denote stereochemistry where appropriate.



Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
10
Provide detailed, arrow-pushing mechanisms for the transformation. Show all reactive intermediates. (It is not necessary to show every resonance structure for appropriate intermediates.)
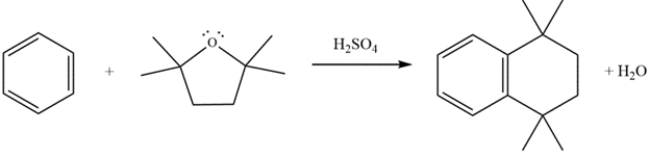


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
11
The -NH2 group is an ortho/para-director. So, explain why the reaction is observed experimentally. Use structures and 15 words or less.
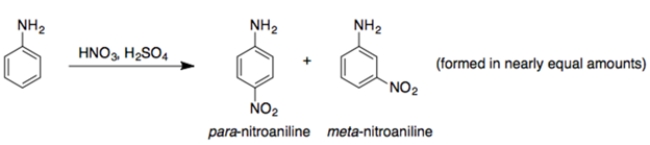


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
12
Indicate whether the compound should undergo nitration more rapidly or more slowly than benzene and give the structure of the major mononitration product. Explain, using a few words and structures.
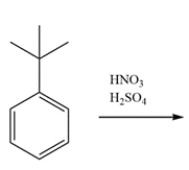


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
13
Indicate whether the compound should undergo nitration more rapidly or more slowly than benzene and give the structure of the major mononitration product. Explain, using a few words and structures.
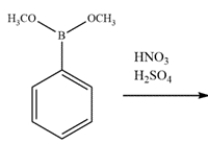


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
14
Devise a two-step synthesis to form the product from benzene. The intermediate is a substituted benzene with a 1H NMR singlet at 2.5 ppm. Identify the intermediate.
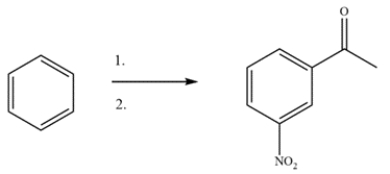


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
15
Draw the major organic product for the reaction.
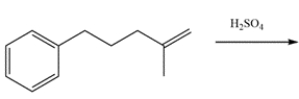


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
16
For each set, circle the faster of the two reactions.
a.
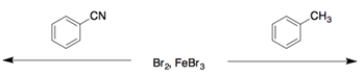
b.
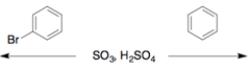
a.
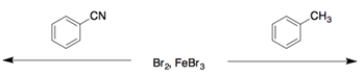
b.


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
17
Indicate whether the compound would undergo nitration more rapidly or more slowly than toluene (also known as methylbenzene) and give the structure of the major mononitration product. Explain, using a few words and structures.
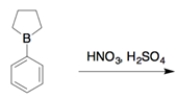


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
18
Indicate whether the compound would undergo nitration more rapidly or more slowly than toluene (also known as methylbenzene) and give the structure of the major mononitration product. Explain, using a few words and structures.
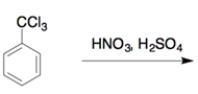


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
19
Write a mechanism for this reaction. Show the formation of major product (in ortho and para situations, just show one, not both). Using structures, and 10 words or less, explain clearly why substitution occurs in the position you indicated over any of the other positions.
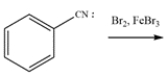


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
20
Write a mechanism for this reaction. Show the formation of major product (in ortho and para situations, just show one, not both). Using structures, and 10 words or less, explain clearly why substitution occurs in the position you indicated over any of the other positions.


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
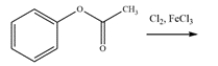
21
Draw the major organic product for the reaction. The product should be one compound with 11 carbons.


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
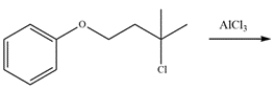
22
Explain how you would distinguish between three isomers with the molecular formula C9H12 using only 1H NMR spectroscopy.


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
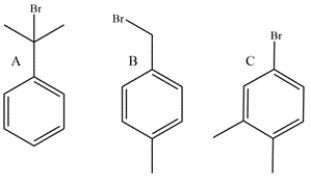
23
Deduce the structure of an unknown compound with the molecular formula C10H14O and the following proton NMR data:
7.39 (d, 2H, J = 7 Hz), 6.93 (d, 2H, J = 7 Hz), 3.81 (s, 3H), 2.87 (septet, 1H, J = 6.8 Hz), 1.20 (d, 6H, J = 6.8 Hz).
7.39 (d, 2H, J = 7 Hz), 6.93 (d, 2H, J = 7 Hz), 3.81 (s, 3H), 2.87 (septet, 1H, J = 6.8 Hz), 1.20 (d, 6H, J = 6.8 Hz).

Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck
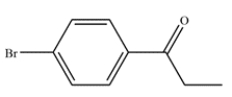
24
Outline a synthesis of this compound from benzene.


Unlock Deck
Unlock for access to all 24 flashcards in this deck.
Unlock Deck
k this deck


