Exam 16: The Chemistry of Benzene and Its Derivatives
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
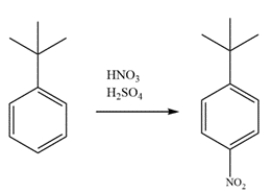
Indicate whether the compound should undergo nitration more rapidly or more slowly than benzene and give the structure of the major mononitration product. Explain, using a few words and structures.
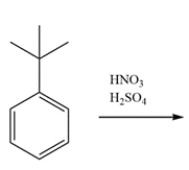
Free
(Essay)
4.9/5  (41)
(41)
Correct Answer:
The benzene ring has an alkyl substituent, so it will react faster than benzene. An alkyl group is an ortho/para director. The para isomer will predominate over the ortho isomer due to the steric hindrance of the bulky tert-butyl group.
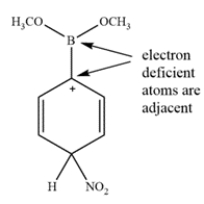
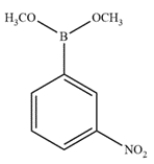
Indicate whether the compound should undergo nitration more rapidly or more slowly than benzene and give the structure of the major mononitration product. Explain, using a few words and structures.
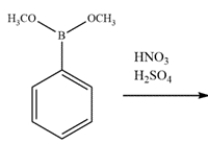
Free
(Essay)
4.9/5  (29)
(29)
Correct Answer:
The boron is inductively withdrawing and destabilizing, so it will react slower than benzene. It will be a meta director since ortho/para substitution would give a very unstable intermediate.
 The major product is:
The major product is:
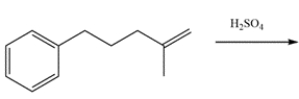
Draw the major organic product for the reaction.
Free
(Essay)
4.8/5  (32)
(32)
Correct Answer:
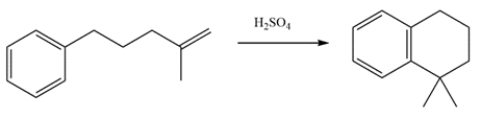
This will be an intramolecular Friedel-Crafts alkylation. The first step is protonation of the alkene by sulfuric acid to form a tertiary carbocation. The carbocation will then react with the ring, then the benzene ring will be regenerated.
Deduce the structure of an unknown compound with the molecular formula C10H14O and the following proton NMR data:
7.39 (d, 2H, J = 7 Hz), 6.93 (d, 2H, J = 7 Hz), 3.81 (s, 3H), 2.87 (septet, 1H, J = 6.8 Hz), 1.20 (d, 6H, J = 6.8 Hz).
(Essay)
4.7/5  (41)
(41)
Provide detailed, arrow-pushing mechanisms for the transformation. Show all reactive intermediates. (It is not necessary to show every resonance structure for appropriate intermediates.)
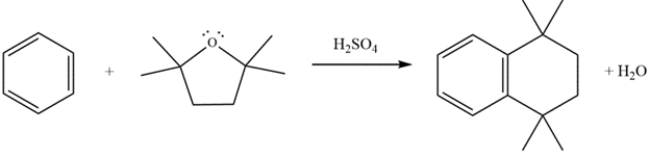
(Essay)
4.8/5  (41)
(41)
Devise a two-step synthesis to form the product from benzene. The intermediate is a substituted benzene with a 1H NMR singlet at 2.5 ppm. Identify the intermediate.
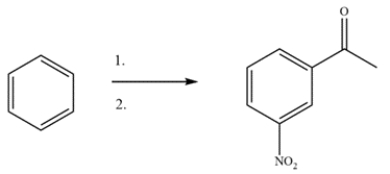
(Essay)
4.8/5  (33)
(33)
Which of these statements is not true about the electrophilic bromination of benzene?
(Multiple Choice)
4.8/5  (38)
(38)
Write a mechanism for this reaction. Show the formation of major product (in ortho and para situations, just show one, not both). Using structures, and 10 words or less, explain clearly why substitution occurs in the position you indicated over any of the other positions.
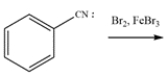
(Essay)
4.8/5  (30)
(30)
Draw the major organic product for the reaction. The product should be one compound with 11 carbons.
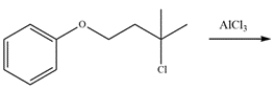
(Essay)
4.8/5  (40)
(40)
Which compound would be the most reactive towards ring bromination?
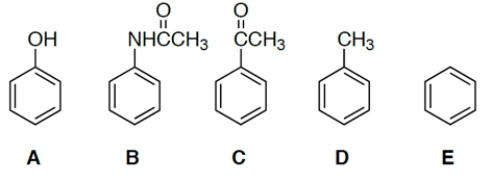
(Multiple Choice)
4.9/5  (29)
(29)
Write a mechanism for this reaction. Show the formation of major product (in ortho and para situations, just show one, not both). Using structures, and 10 words or less, explain clearly why substitution occurs in the position you indicated over any of the other positions.
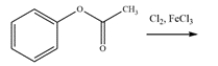
(Essay)
4.7/5  (41)
(41)
Explain how you would distinguish between three isomers with the molecular formula C9H12 using only 1H NMR spectroscopy.
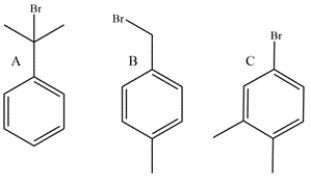
(Essay)
4.8/5  (38)
(38)
The -NH2 group is an ortho/para-director. So, explain why the reaction is observed experimentally. Use structures and 15 words or less.
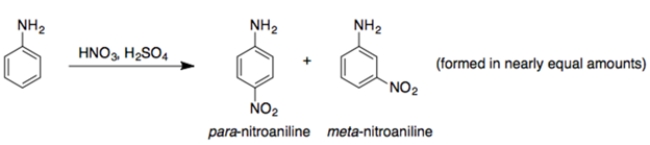
(Essay)
4.9/5  (31)
(31)
A reaction forms the ortho, meta, and para isomers of dibromobenzene. Draw the structures of the three compounds and explain how you can use the melting point to separate the mixtures. Which would you expect to have the highest melting point?
(Essay)
4.8/5  (40)
(40)
Indicate the missing products of the reaction. When filling in the product indicate only the major organic product(s). There may be more than one acceptable answer. If you believe no reaction would occur, write NR. Denote stereochemistry where appropriate.

(Essay)
4.8/5  (41)
(41)
Showing 1 - 20 of 24
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)