Deck 14: Nuclear Magnetic Resonance Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/27
Play
Full screen (f)
Deck 14: Nuclear Magnetic Resonance Spectroscopy
1
Which letter corresponds to the set of protons that experience the greatest magnetic field when a sample of the compound is placed in the probe of an NMR spectrometer?
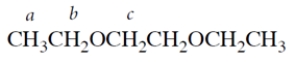
A) a
B) b
C) c
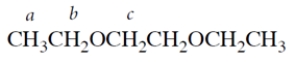
A) a
B) b
C) c
C
2
Consider the labeled protons in the compound.
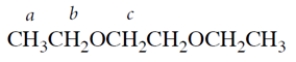 The resonance of protons c would appear in the NMR spectrum as
The resonance of protons c would appear in the NMR spectrum as
A) a singlet.
B) a doublet.
C) a triplet.
D) a quartet.
E) an octet (an eight-line splitting pattern).f.
some other splitting pattern.
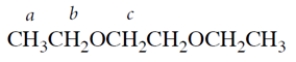 The resonance of protons c would appear in the NMR spectrum as
The resonance of protons c would appear in the NMR spectrum asA) a singlet.
B) a doublet.
C) a triplet.
D) a quartet.
E) an octet (an eight-line splitting pattern).f.
some other splitting pattern.
A
3
Of the protons that are labeled, which set experiences the greatest magnetic field in an NMR spectrometer?
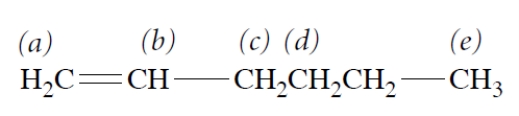
A) a
B) b
C) c
D) d
E) e
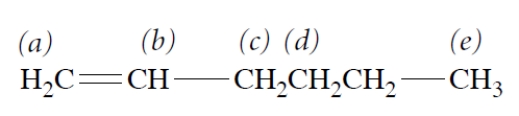
A) a
B) b
C) c
D) d
E) e
B
4
The resonance for the methyl group in 1-bromo-1-chloroethane (CH3-CHBrCl) is
A) a singlet.
B) a doublet.
C) a triplet.
D) two doublets because the protons are diastereotopic.
E) none of these choices.
A) a singlet.
B) a doublet.
C) a triplet.
D) two doublets because the protons are diastereotopic.
E) none of these choices.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
5
In an NMR spectrometer operating at ν0 = 300 MHz, a resonance that has a chemical shift of 2.0 ppm (i.e., 2) occurs at a frequency that is
A) 600 Hz greater than the absorption frequency of the standard TMS.
B) 600 × 106 Hz greater than the absorption frequency of TMS
C) 150 Hz greater than the absorption frequency of TMS.
D) 150,000,000 Hz greater than the absorption frequency of TMS.
E) 302 Hz greater than the absorption frequency of TMS.f.
less than the absorption frequency of TMS.
A) 600 Hz greater than the absorption frequency of the standard TMS.
B) 600 × 106 Hz greater than the absorption frequency of TMS
C) 150 Hz greater than the absorption frequency of TMS.
D) 150,000,000 Hz greater than the absorption frequency of TMS.
E) 302 Hz greater than the absorption frequency of TMS.f.
less than the absorption frequency of TMS.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the compound.
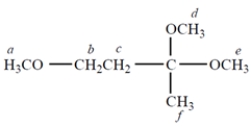 a. How many separate singlets (unsplit NMR resonances) are there in the NMR spectrum of this compound?
a. How many separate singlets (unsplit NMR resonances) are there in the NMR spectrum of this compound?
b. The resonance that is at the highest frequency (farthest to the left) is
1. a.
2. b.
3. c.
4. d and/or e.
5. f.
c. The resonance of protons c appears as
1. a singlet.
2. a doublet.
3. a triplet.
4. a very complex pattern because the protons are diastereotopic.
 a. How many separate singlets (unsplit NMR resonances) are there in the NMR spectrum of this compound?
a. How many separate singlets (unsplit NMR resonances) are there in the NMR spectrum of this compound?b. The resonance that is at the highest frequency (farthest to the left) is
1. a.
2. b.
3. c.
4. d and/or e.
5. f.
c. The resonance of protons c appears as
1. a singlet.
2. a doublet.
3. a triplet.
4. a very complex pattern because the protons are diastereotopic.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
7
7. Consider the NMR spectrum of the compound:
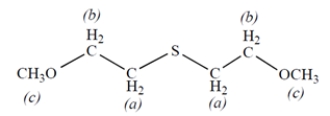
-The protons labeled ________________.
A) should have the smallest chemical shift in the spectrum
B) should have the greatest chemical shift in the spectrum
C) should have neither the smallest nor the greatest chemical shift in the spectrum

-The protons labeled ________________.
A) should have the smallest chemical shift in the spectrum
B) should have the greatest chemical shift in the spectrum
C) should have neither the smallest nor the greatest chemical shift in the spectrum

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
8
7. Consider the NMR spectrum of the compound:

-The resonance for protons (a) is ________________.
A) a singlet
B) a doublet
C) a triplet
D) a quartet
E) more complex

-The resonance for protons (a) is ________________.
A) a singlet
B) a doublet
C) a triplet
D) a quartet
E) more complex

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
9
Which compound contains two three-proton singlets in its NMR spectrum?
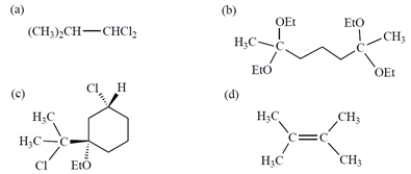
A) compound a
B) compound b
C) compound c
D) compound d

A) compound a
B) compound b
C) compound c
D) compound d

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
10
What radiation frequency (in sec-1, or Hz) is required to bring a set of protons into resonance if they experience a magnetic field of 50,000 gauss? Show your work.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the bolded protons in 1-propanol.
Following a "D2O shake," the splitting of the 1-propanol protons shown in bold type should be observed as
A) a singlet.
B) a doublet.
C) a triplet.
D) a quartet.
E) some other splitting.
Following a "D2O shake," the splitting of the 1-propanol protons shown in bold type should be observed as
A) a singlet.
B) a doublet.
C) a triplet.
D) a quartet.
E) some other splitting.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
12
11. Consider the ether:
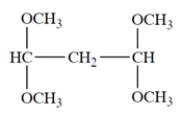
-How many resonances should there be in the NMR spectrum of the compound? (A split resonance counts as one resonance.)

-How many resonances should there be in the NMR spectrum of the compound? (A split resonance counts as one resonance.)

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
13
11. Consider the ether:

-The resonance for the CH2 hydrogens in the structure .is: (circle ONE)
A) two doublets.
B) two triplets.
C) one triplet.
D) one singlet.
E) some other pattern.

-The resonance for the CH2 hydrogens in the structure .is: (circle ONE)
A) two doublets.
B) two triplets.
C) one triplet.
D) one singlet.
E) some other pattern.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
14
A compound X has a formula C7H16O4 and the following NMR spectrum, given as chemical shift (integral, splitting, coupling constant).
1.93 (2H, t, J = 6 Hz); 3.35 (12H, s); 4.49 (2H, t, J = 6 Hz)
Which structure is consistent with this NMR spectrum?
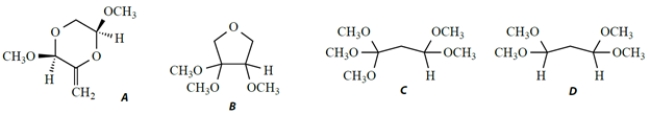
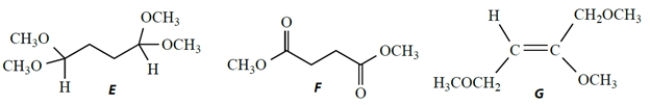
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E
F) compound F
G) compound G
1.93 (2H, t, J = 6 Hz); 3.35 (12H, s); 4.49 (2H, t, J = 6 Hz)
Which structure is consistent with this NMR spectrum?


A) compound A
B) compound B
C) compound C
D) compound D
E) compound E
F) compound F
G) compound G

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
15
Consider how the NMR spectrum of protons a in the compound changes on the addition of a trace of acid (assume that Jab ≠ Jac):
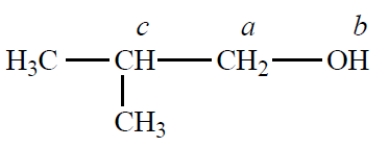
A) no change
B) from a doublet of doublets to a simple doublet
C) from a triplet to a singlet
D) from a doublet of doublets to a triplet
E) none of these choices
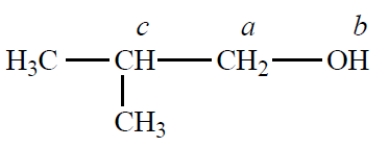
A) no change
B) from a doublet of doublets to a simple doublet
C) from a triplet to a singlet
D) from a doublet of doublets to a triplet
E) none of these choices

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
16
Deduce a structure that corresponds to the molecular formula and NMR spectra. Explain how you arrived at this structure.
Molecular formula: C5H12O.
1H NMR: 1.18 (3H, t, J = 8 Hz); 1.13 (6H, d, J = 6.8 Hz); 3.53 (2H, q, J = 8.0 Hz); 3.65 (1H, septet, J = 6.8 Hz).
Molecular formula: C5H12O.
1H NMR: 1.18 (3H, t, J = 8 Hz); 1.13 (6H, d, J = 6.8 Hz); 3.53 (2H, q, J = 8.0 Hz); 3.65 (1H, septet, J = 6.8 Hz).

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
17
How many signals would you expect in a 13C NMR spectrum for this compound? Identify the carbon with the highest chemical shift.
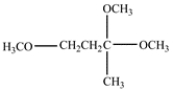


Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
18
Explain how you could use proton decoupled 13C NMR to differentiate between these two constitutional isomers?
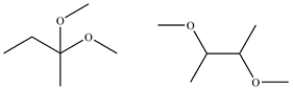


Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
19
Deduce the structure of a compound with a molecular formula C8H10 that gives the proton decoupled 13C NMR data: 21.3, 128.9, 135.4 ppm.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
20
Deduce the structure of an unknown compound with a molecular formula of C4H8O given the following 13C NMR - DEPT spectrum. The numbers in parentheses indicate the number of attached hydrogens.
Proton decoupled 13C NMR: 23.3 (3), 68.5 (1), 116.4 (2), 135.7 (1)
Proton decoupled 13C NMR: 23.3 (3), 68.5 (1), 116.4 (2), 135.7 (1)

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
21
a. Identify compound X with the formula C5H10O that has the IR and NMR spectra shown.
b. Compound X is subjected to catalytic hydrogenation (H2, Pd/C) to give compound Y. Give the structure of compound Y and describe the most significant difference between the NMR spectra of X and Y.
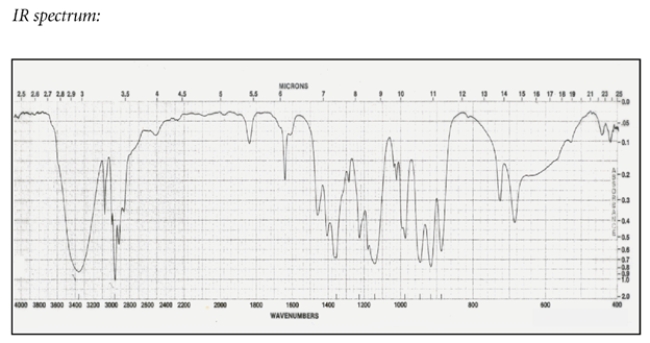
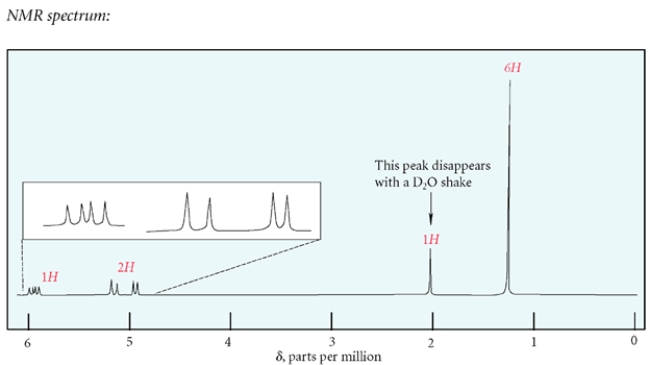
b. Compound X is subjected to catalytic hydrogenation (H2, Pd/C) to give compound Y. Give the structure of compound Y and describe the most significant difference between the NMR spectra of X and Y.



Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
22
Deduce the structure of the unknown compound C6H12O, whose IR and NMR spectra are shown. Explain how you arrived at your structure.
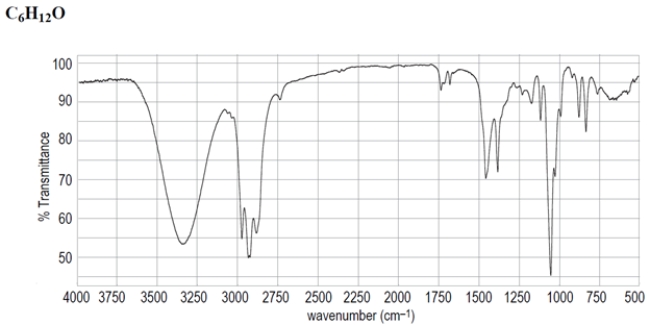
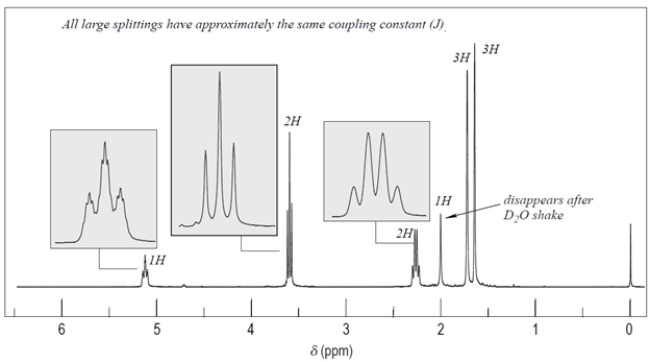



Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
23
A compound X with the formula C4H10O2 has the NMR spectrum shown. The integrals are given over their respective resonances, and the J values are coupling constants. Deduce the structure of compound X and explain how you came to this conclusion. Hints: (1) The only chemical shift information you need is that protons on carbons to an oxygen have chemical shifts in the 3.2- 4.0 range. (2) You do not need to interpret the complex splittings at 3.22, 3.37, and 3.9 to deduce the structure, but you will need to interpret the splittings in the gray boxes.
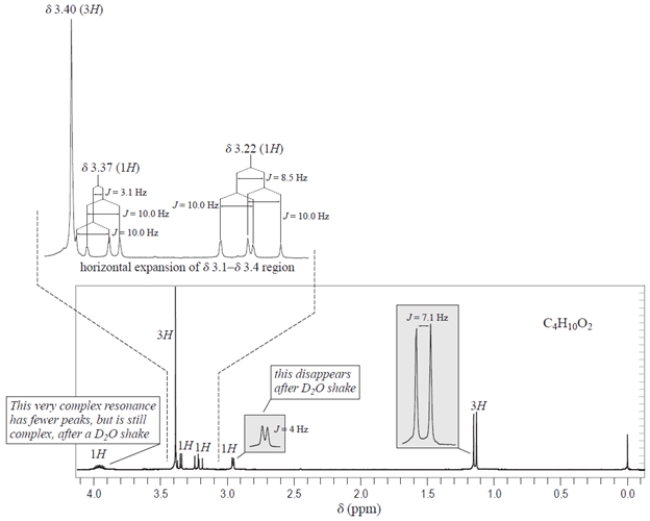


Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
24
Deduce the structure of a compound C6H14O that corresponds to the proton NMR spectrum. The integral numbers are relative numbers of hydrogens. Assign each peak. There are no absorptions in the 3200-3600 cm-1 region of the IR.
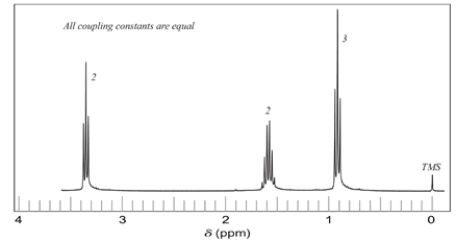


Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
25
The NMR spectrum of the missing compound is given below the reaction. Draw the structure of the missing starting material.
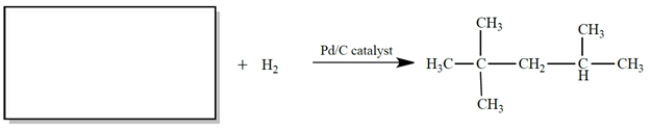
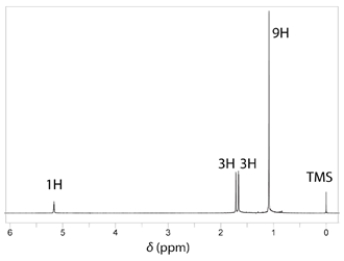



Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
26
The spectra were recorded for a compound with a molecular formula C5H10O2. Deduce the structure of this compound and explain how you arrive at the correct structure of the compound. (Hint: There are diastereotopic protons in this compound.) NOTE: In the NMR, after a D2O shake, the triplet at 3.27 disappears, and the doublet at 3.7 becomes a singlet.
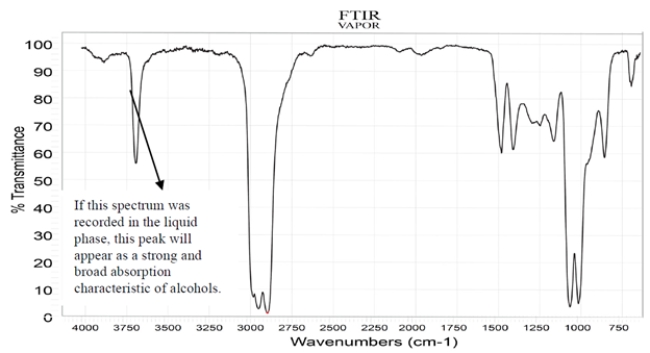
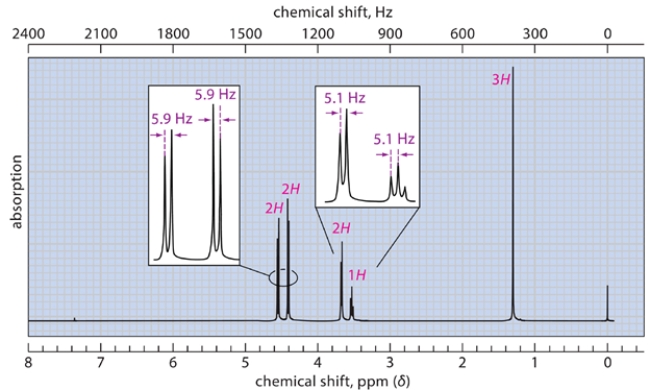



Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
27
An unknown compound has a molecular formula of C5H8. Deduce the structure by using the proton and carbon NMR spectra provided.
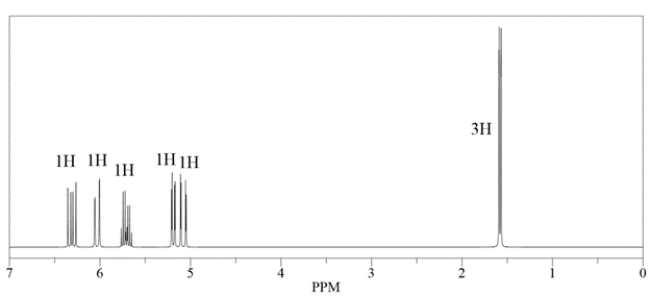
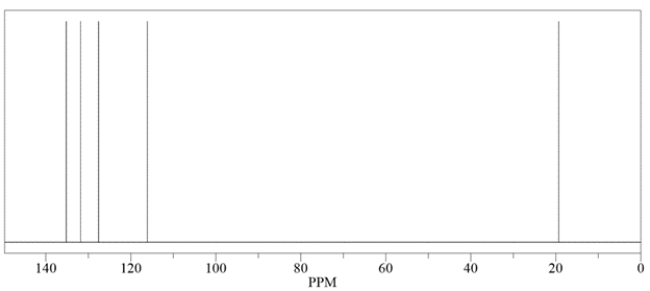



Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck


