Exam 14: Nuclear Magnetic Resonance Spectroscopy
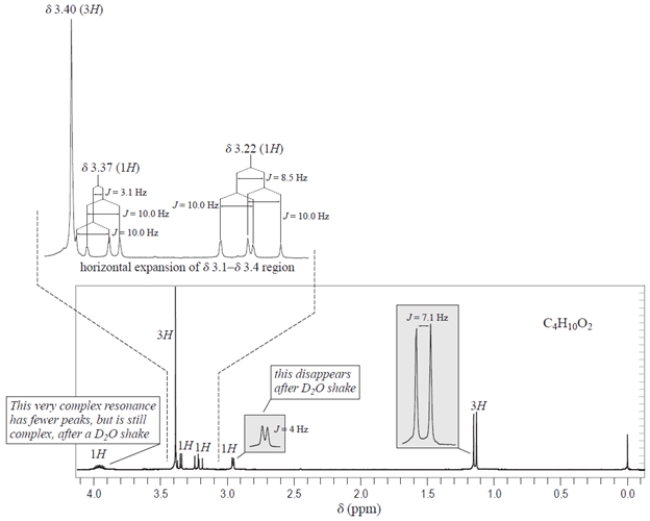
Which compound contains two three-proton singlets in its NMR spectrum?
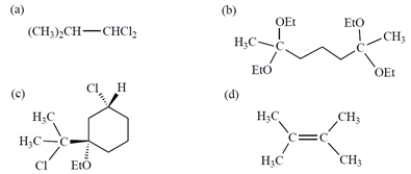
C
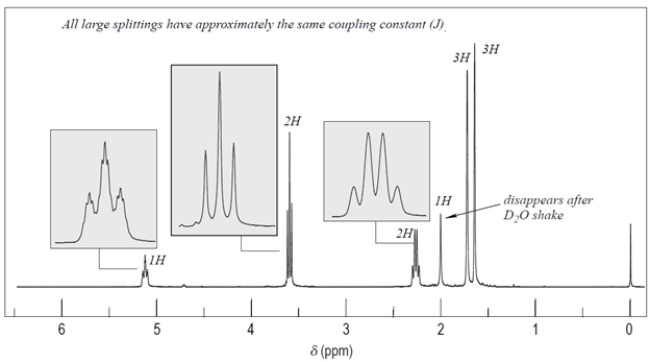
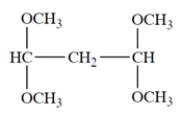
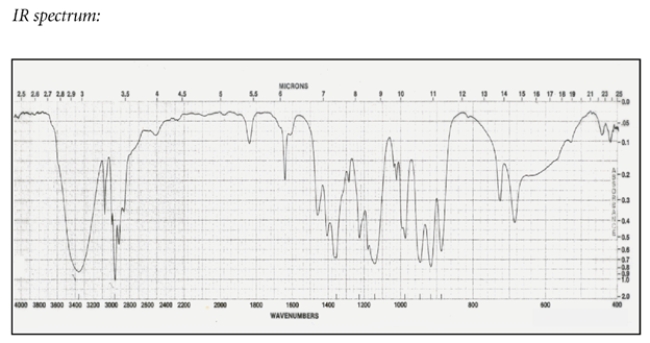
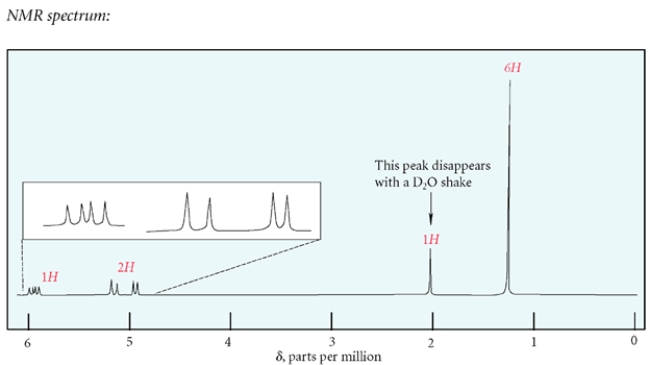
Deduce the structure of the unknown compound C6H12O, whose IR and NMR spectra are shown. Explain how you arrived at your structure.
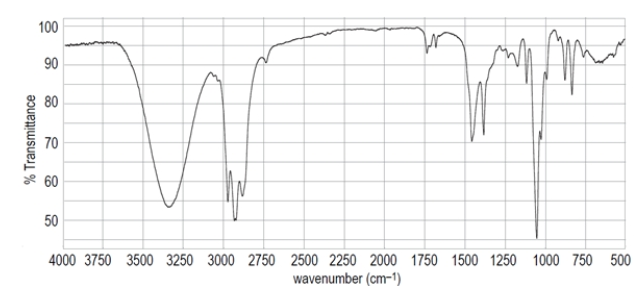
First calculate the degree of unsaturation from the molecular formula. Recall the formula:
The unsaturation number is equal to (2*6+2-12)/2 = 1, so there is at least one double bond or ring. The IR spectrum shows a diagnostic peak for hydroxy, but not much else. The NMR spectra confirms the presence of the hydroxy group with a peak at 2.0 ppm that disappears after a D2O shake. The NMR spectra also show two inequivalent methyl groups, two CH2 groups and an alkene proton. Since there is only one alkene proton, the unknown compound must be a trisubstituted alkene. The chemical shift of the CH2 group at 3.6 ppm suggests it is near the hydroxy group, giving the fragment -CH2OH. Putting the fragments together, the structure must be:
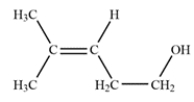 Let's check the structure and make sure it matches the data. The two methyl groups are diastereotopic, so they will not be equivalent. They do not have any adjacent protons, so they are singlets. The CH2 group bonded to the alkene is split by the alkene proton and the adjacent methylene to give an apparent quartet. The alkene is split by the CH2 group, but also by some long-range coupling interactions by protons transmitted by the pi electrons. The remaining CH2 group is expected to be a triplet and the hydroxy proton is a singlet, as expected.
Let's check the structure and make sure it matches the data. The two methyl groups are diastereotopic, so they will not be equivalent. They do not have any adjacent protons, so they are singlets. The CH2 group bonded to the alkene is split by the alkene proton and the adjacent methylene to give an apparent quartet. The alkene is split by the CH2 group, but also by some long-range coupling interactions by protons transmitted by the pi electrons. The remaining CH2 group is expected to be a triplet and the hydroxy proton is a singlet, as expected.
Consider the compound.
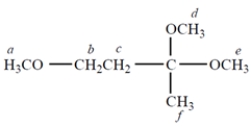 a. How many separate singlets (unsplit NMR resonances) are there in the NMR spectrum of this compound?
b. The resonance that is at the highest frequency (farthest to the left) is
1. a.
2. b.
3. c.
4. d and/or e.
5. f.
c. The resonance of protons c appears as
1. a singlet.
2. a doublet.
3. a triplet.
4. a very complex pattern because the protons are diastereotopic.
a. How many separate singlets (unsplit NMR resonances) are there in the NMR spectrum of this compound?
b. The resonance that is at the highest frequency (farthest to the left) is
1. a.
2. b.
3. c.
4. d and/or e.
5. f.
c. The resonance of protons c appears as
1. a singlet.
2. a doublet.
3. a triplet.
4. a very complex pattern because the protons are diastereotopic.
a. There are three singlets; protons d and e are equivalent. Thus, protons a, d + e, and f are the three singlets.
b. The candidates would be a, d, e, and b because these protons are closest to an oxygen. Because methylene protons have a greater chemical shift than methyl protons with the same adjacent atom, the answer is b.
c. Protons c appear as a triplet. (They are enantiotopic and thus equivalent; they are adjacent to two protons, for which the n + 1 rule gives a triplet.)
Deduce a structure that corresponds to the molecular formula and NMR spectra. Explain how you arrived at this structure.
Molecular formula: C5H12O.
1H NMR: 1.18 (3H, t, J = 8 Hz); 1.13 (6H, d, J = 6.8 Hz); 3.53 (2H, q, J = 8.0 Hz); 3.65 (1H, septet, J = 6.8 Hz).
In an NMR spectrometer operating at ν0 = 300 MHz, a resonance that has a chemical shift of 2.0 ppm (i.e., 2) occurs at a frequency that is
What radiation frequency (in sec-1, or Hz) is required to bring a set of protons into resonance if they experience a magnetic field of 50,000 gauss? Show your work.
7. Consider the NMR spectrum of the compound:
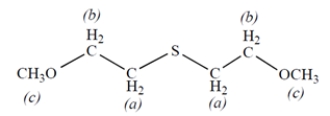 -The protons labeled ________________.
-The protons labeled ________________.
An unknown compound has a molecular formula of C5H8. Deduce the structure by using the proton and carbon NMR spectra provided.
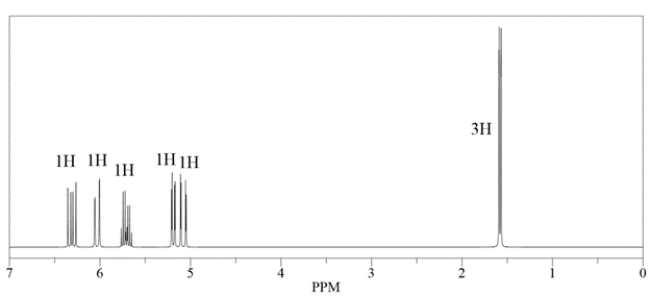
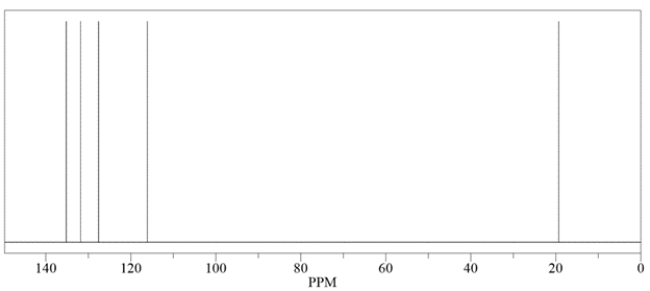
How many signals would you expect in a 13C NMR spectrum for this compound? Identify the carbon with the highest chemical shift.
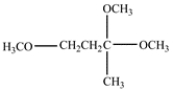
A compound X with the formula C4H10O2 has the NMR spectrum shown. The integrals are given over their respective resonances, and the J values are coupling constants. Deduce the structure of compound X and explain how you came to this conclusion. Hints: (1) The only chemical shift information you need is that protons on carbons to an oxygen have chemical shifts in the 3.2- 4.0 range. (2) You do not need to interpret the complex splittings at 3.22, 3.37, and 3.9 to deduce the structure, but you will need to interpret the splittings in the gray boxes.
Of the protons that are labeled, which set experiences the greatest magnetic field in an NMR spectrometer?
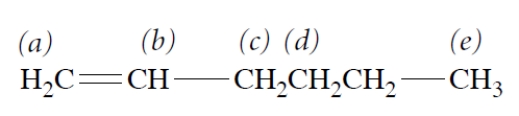
Consider how the NMR spectrum of protons a in the compound changes on the addition of a trace of acid (assume that Jab ≠ Jac):
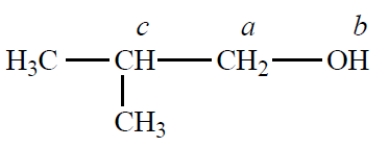
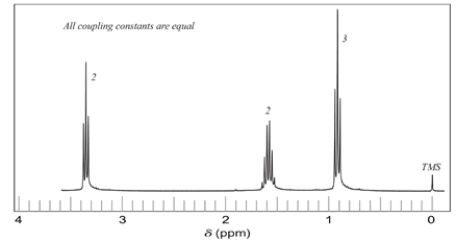
11. Consider the ether:
 -How many resonances should there be in the NMR spectrum of the compound? (A split resonance counts as one resonance.)
-How many resonances should there be in the NMR spectrum of the compound? (A split resonance counts as one resonance.)
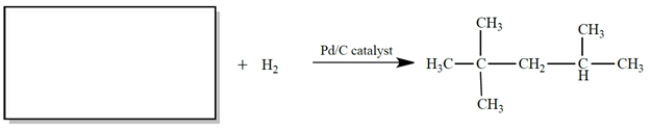
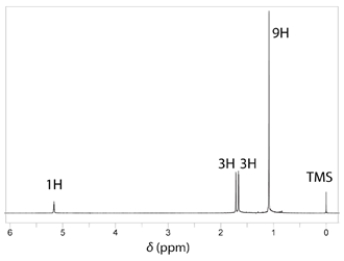
a. Identify compound X with the formula C5H10O that has the IR and NMR spectra shown.
b. Compound X is subjected to catalytic hydrogenation (H2, Pd/C) to give compound Y. Give the structure of compound Y and describe the most significant difference between the NMR spectra of X and Y.
Consider the labeled protons in the compound.
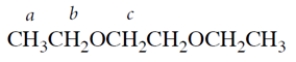 The resonance of protons c would appear in the NMR spectrum as
The resonance of protons c would appear in the NMR spectrum as
Deduce the structure of an unknown compound with a molecular formula of C4H8O given the following 13C NMR - DEPT spectrum. The numbers in parentheses indicate the number of attached hydrogens.
Proton decoupled 13C NMR: 23.3 (3), 68.5 (1), 116.4 (2), 135.7 (1)
The NMR spectrum of the missing compound is given below the reaction. Draw the structure of the missing starting material.
Which letter corresponds to the set of protons that experience the greatest magnetic field when a sample of the compound is placed in the probe of an NMR spectrometer?
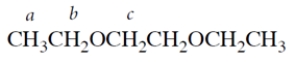
Consider the bolded protons in 1-propanol.
Following a "D2O shake," the splitting of the 1-propanol protons shown in bold type should be observed as
Deduce the structure of a compound C6H14O that corresponds to the proton NMR spectrum. The integral numbers are relative numbers of hydrogens. Assign each peak. There are no absorptions in the 3200-3600 cm-1 region of the IR.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)