Deck 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides
1
Provide the major organic product for the reaction sequence:
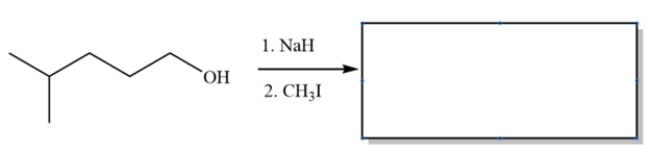

This is an example of the Williamson ether synthesis. The first step deprotonates the alcohol to form an alkoxide. The second step is simply an SN2 substitution.

2
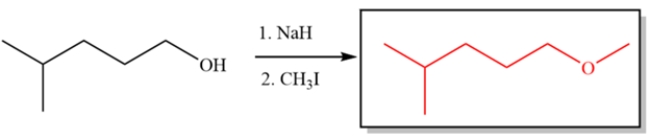
The reaction fails to generate the indicated ether. Explain why and what product is generated instead.
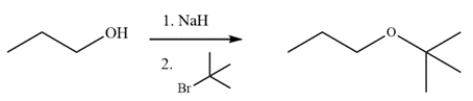

The alkoxide will be generated in the first step, but a strong base with a tertiary alkyl halide will lead to elimination, not substitution. The product generated will be 2-methyl-1-propene instead.
3
Outline the synthesis of dicyclopentyl ether using alkoxymercuration-reduction.
This ether can be generated by treatment of cyclopentene with cyclopentanol and Hg(OAc)2, followed by reduction with NaBH4.
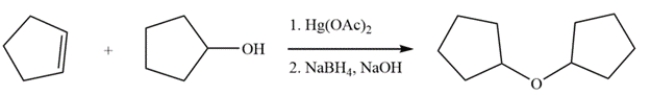
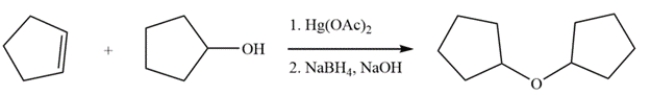
4
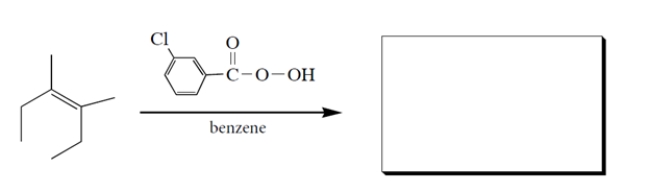
Provide the missing product. Clearly show stereochemistry of the product.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
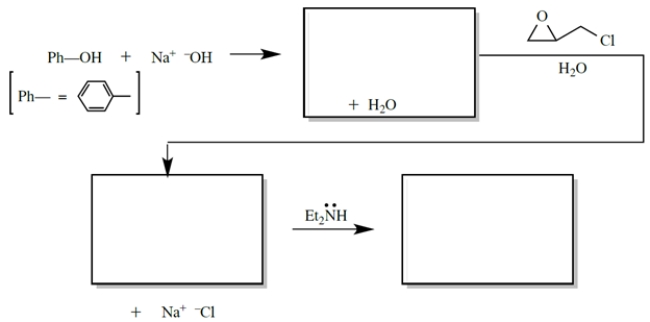
Complete the reaction sequence by providing the structures of the missing compounds.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
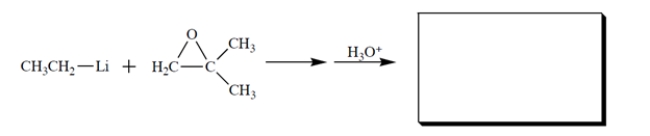
Provide the missing product and the curved-arrow notation for this reaction.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
Benzo[a]pyrene is a carcinogenic polyaromatic hydrocarbon found in cigarette smoke that is converted by enzymes in the body to a diol epoxide.
![Benzo[a]pyrene is a carcinogenic polyaromatic hydrocarbon found in cigarette smoke that is converted by enzymes in the body to a diol epoxide. This diol epoxide product can react with nucleophiles on DNA and thus promote cancer. (This is one of the main reasons that cigarette smoke is carcinogenic.) The body can neutralize this toxin by reacting it with glutathione, a thiol. Representing glutathione as R-SH, show two products that might form when glutathione reacts with the diol epoxide, including their stereochemistry. Although acids and bases for this reaction are typically provided by the enzymes that catalyze this transformation, assume that -OH is the base and H<sub>2</sub>O is the acid where acids and bases are necessary.](https://storage.examlex.com/TBMC1048/11edaded_7d3b_35c7_a31a_79fa30fdaebc_TBMC1048_00.jpg) This diol epoxide product can react with nucleophiles on DNA and thus promote cancer. (This is one of the main reasons that cigarette smoke is carcinogenic.) The body can neutralize this toxin by reacting it with glutathione, a thiol. Representing glutathione as R-SH, show two products that might form when glutathione reacts with the diol epoxide, including their stereochemistry. Although acids and bases for this reaction are typically provided by the enzymes that catalyze this transformation, assume that -OH is the base and H2O is the acid where acids and bases are necessary.
This diol epoxide product can react with nucleophiles on DNA and thus promote cancer. (This is one of the main reasons that cigarette smoke is carcinogenic.) The body can neutralize this toxin by reacting it with glutathione, a thiol. Representing glutathione as R-SH, show two products that might form when glutathione reacts with the diol epoxide, including their stereochemistry. Although acids and bases for this reaction are typically provided by the enzymes that catalyze this transformation, assume that -OH is the base and H2O is the acid where acids and bases are necessary.
![Benzo[a]pyrene is a carcinogenic polyaromatic hydrocarbon found in cigarette smoke that is converted by enzymes in the body to a diol epoxide. This diol epoxide product can react with nucleophiles on DNA and thus promote cancer. (This is one of the main reasons that cigarette smoke is carcinogenic.) The body can neutralize this toxin by reacting it with glutathione, a thiol. Representing glutathione as R-SH, show two products that might form when glutathione reacts with the diol epoxide, including their stereochemistry. Although acids and bases for this reaction are typically provided by the enzymes that catalyze this transformation, assume that -OH is the base and H<sub>2</sub>O is the acid where acids and bases are necessary.](https://storage.examlex.com/TBMC1048/11edaded_7d3b_35c7_a31a_79fa30fdaebc_TBMC1048_00.jpg) This diol epoxide product can react with nucleophiles on DNA and thus promote cancer. (This is one of the main reasons that cigarette smoke is carcinogenic.) The body can neutralize this toxin by reacting it with glutathione, a thiol. Representing glutathione as R-SH, show two products that might form when glutathione reacts with the diol epoxide, including their stereochemistry. Although acids and bases for this reaction are typically provided by the enzymes that catalyze this transformation, assume that -OH is the base and H2O is the acid where acids and bases are necessary.
This diol epoxide product can react with nucleophiles on DNA and thus promote cancer. (This is one of the main reasons that cigarette smoke is carcinogenic.) The body can neutralize this toxin by reacting it with glutathione, a thiol. Representing glutathione as R-SH, show two products that might form when glutathione reacts with the diol epoxide, including their stereochemistry. Although acids and bases for this reaction are typically provided by the enzymes that catalyze this transformation, assume that -OH is the base and H2O is the acid where acids and bases are necessary.
Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Complete the reaction by giving the major organic product:
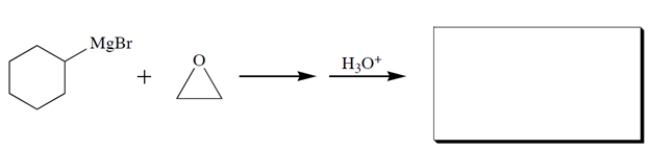


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Complete the missing starting material. Be sure to clearly show stereochemistry.
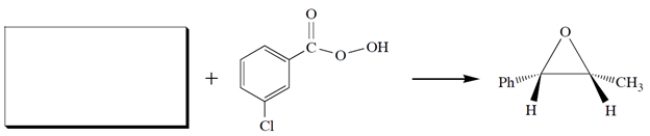


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Complete the reaction by giving the major organic product. The product formed is a racemate-give the stereochemistry of either enantiomer.
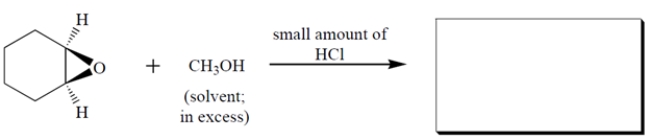


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
Complete the reaction:
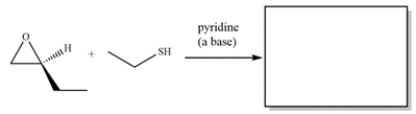


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Outline a synthesis to give the transformation:
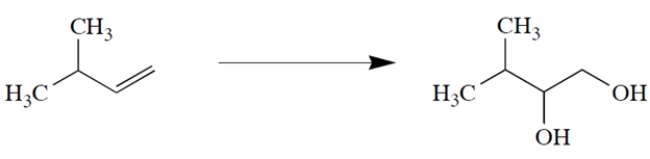


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
cis-3-Hexene is treated with OsO4 (osmium tetroxide), then water. Which one statement is true about the product?
A) The product is the meso stereoisomer of 3,4-hexanediol, and it is optically inactive.
B) The product is mostly the (3S,4R) stereoisomer of 3,4-hexanediol, and it is optically active.
C) The product is mostly optically active (3S,4S)-3,4-hexanediol.
D) The product is mostly optically active (3R,4R)-3,4-hexanediol.
E) The product is a racemate consisting of equal amounts of (3S,4S)-3,4-hexanediol and (3R,4R)-3,4-hexanediol.f.
None of the these is correct because 3,4-hexanediol is not the product of this reaction.
A) The product is the meso stereoisomer of 3,4-hexanediol, and it is optically inactive.
B) The product is mostly the (3S,4R) stereoisomer of 3,4-hexanediol, and it is optically active.
C) The product is mostly optically active (3S,4S)-3,4-hexanediol.
D) The product is mostly optically active (3R,4R)-3,4-hexanediol.
E) The product is a racemate consisting of equal amounts of (3S,4S)-3,4-hexanediol and (3R,4R)-3,4-hexanediol.f.
None of the these is correct because 3,4-hexanediol is not the product of this reaction.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
In the SN2 reaction, sulfur is the "leaving atom" in the leaving group. The products are a single sulfide (R-S-R´) and a single ether.
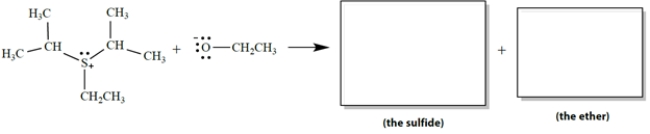 a. Draw the structures of the products in the appropriate boxes.
a. Draw the structures of the products in the appropriate boxes.
b. On the reaction, draw the curved-arrow notation for this reaction.
 a. Draw the structures of the products in the appropriate boxes.
a. Draw the structures of the products in the appropriate boxes.b. On the reaction, draw the curved-arrow notation for this reaction.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Give the structure of the nucleophile that would react with benzyl chloride (PhCH2Cl) to give the sulfonium salt:
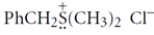
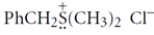

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
Select the one true statement about biological reactions involving S-adenosylmethionine (SAM).
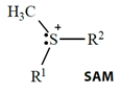
A) The sulfur acts as a nucleophile.
B) The sulfur acts as a nucleophile in scavenging unwanted epoxides.
C) The sulfur (with its two attached groups) acts as a leaving group.
D) The R1 and R2 groups attached to the sulfur act as electrophiles.
E) The methyl group attached to sulfur acts as a nucleophile.

A) The sulfur acts as a nucleophile.
B) The sulfur acts as a nucleophile in scavenging unwanted epoxides.
C) The sulfur (with its two attached groups) acts as a leaving group.
D) The R1 and R2 groups attached to the sulfur act as electrophiles.
E) The methyl group attached to sulfur acts as a nucleophile.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
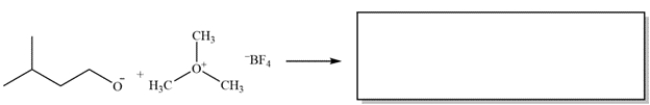
Complete the reaction:


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
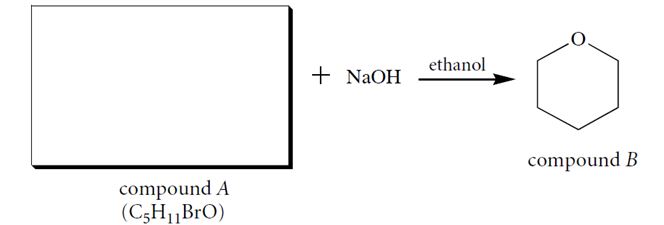
Compound A (C5H11BrO), when dissolved in ethanol containing one equivalent of NaOH, forms a product B. Identify compound A and give a two-step curved-arrow mechanism for its conversion into B. In each step of the curved-arrow mechanism identify the Brønsted acids (BA), Brønsted bases (BB), nucleophiles (N), electrophiles (E), and leaving groups (LG).


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Using curved-arrow notation, propose a mechanism for the transformation that takes into account the stereochemistry of the product. (Hint: Note that two inversions of stereochemistry = one retention.)
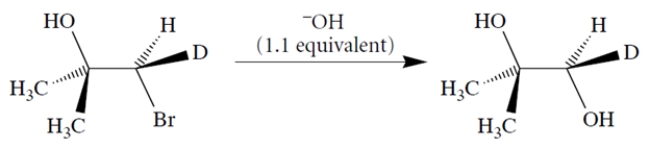
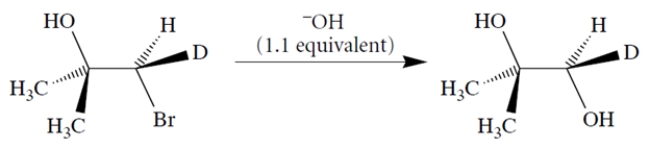

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
a. Only one of the two diastereomers (formula C12H23OBr) will react with NaOH to form a product X with the formula C12H22O. Identify the reactive diastereomer by circling it and labeling it with the letter A. Draw the product X in its chair conformation.
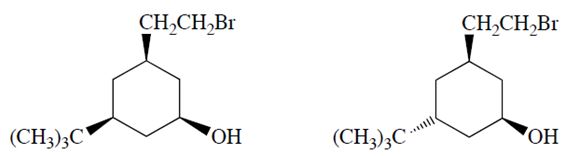 b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.
b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.
c. Explain why compounds A and B react differently and explain why the formation of X from A is much faster than the formation of Y from B.
 b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.
b. The other diastereomer (B) reacts more slowly but eventually forms a product Y (C12H24O2). Compound Y is not an alkene. Propose a structure for Y.c. Explain why compounds A and B react differently and explain why the formation of X from A is much faster than the formation of Y from B.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
Draw the missing organic product.
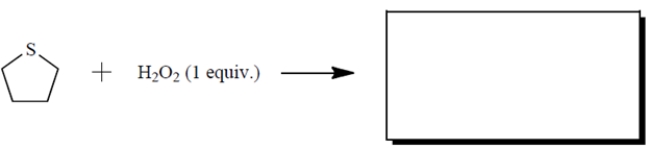


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Propose a synthetic scheme to carry out the transformation. Show the product of each step.
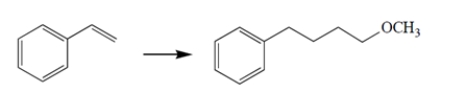


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Propose a multistep synthesis for the epoxide (which will be prepared as the racemate) from 1-butyne, benzyl bromide, and any other reagents. As usual, show the reagents and the major organic product for each step.
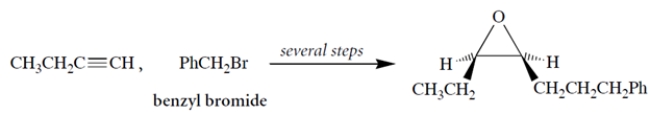


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
Complete the diagram by supplying structures for all the missing compounds.
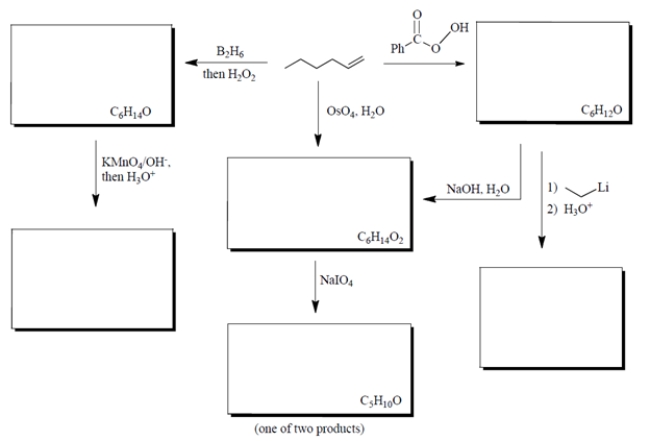


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Outline a multistep synthesis of the given alkene from acetylene and any other reagents.
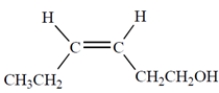 You must use the following reagents at one or more points in your synthesis (as well as other reagents). Note: These are not necessarily used at the same step. Show the organic products (not the reactive intermediates or by-products) resulting from each step.
You must use the following reagents at one or more points in your synthesis (as well as other reagents). Note: These are not necessarily used at the same step. Show the organic products (not the reactive intermediates or by-products) resulting from each step.
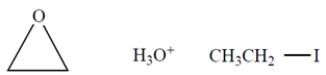
 You must use the following reagents at one or more points in your synthesis (as well as other reagents). Note: These are not necessarily used at the same step. Show the organic products (not the reactive intermediates or by-products) resulting from each step.
You must use the following reagents at one or more points in your synthesis (as well as other reagents). Note: These are not necessarily used at the same step. Show the organic products (not the reactive intermediates or by-products) resulting from each step.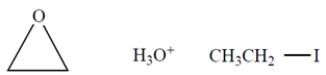

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


