Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides
Exam 1: Chemical Bonding and Chemical Structure26 Questions
Exam 2: Alkanes and Organic Nomenclature25 Questions
Exam 3: The Curved-Arrow Notation, Resonance, Acids and Bases, and Chemical Equilibrium25 Questions
Exam 4: Introduction to Alkenes and Alkynes26 Questions
Exam 5: Addition Reactions of Alkenes and Alkynes25 Questions
Exam 6: Principles of Stereochemistry25 Questions
Exam 7: Cyclic Compounds and Reaction Stereochemistry25 Questions
Exam 8: Nomenclature and Noncovalent Intermolecular Interactions25 Questions
Exam 9: The Chemistry of Alkyl Halides25 Questions
Exam 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes25 Questions
Exam 11: The Chemistry of Alcohols and Thiols25 Questions
Exam 12: The Chemistry of Ethers, Epoxides, Glycols, and Sulfides25 Questions
Exam 13: Introduction to Spectroscopy25 Questions
Exam 14: Nuclear Magnetic Resonance Spectroscopy27 Questions
Exam 15: Dienes and Aromaticity25 Questions
Exam 16: The Chemistry of Benzene and Its Derivatives24 Questions
Exam 17: Allylic and Benzylic Reactivity25 Questions
Exam 18: The Chemistry of Aryl Halides, Vinylic Halides, and Phenols25 Questions
Exam 19: The Chemistry of Aldehydes and Ketones25 Questions
Exam 20: The Chemistry of Carboxylic Acids25 Questions
Exam 21: The Chemistry of Carboxylic Acid Derivatives25 Questions
Exam 22: The Chemistry of Enolate Ions, Enols, and Α,β-Unsaturated Carbonyl Compounds25 Questions
Exam 23: The Chemistry of Amines25 Questions
Exam 24: Carbohydrates25 Questions
Exam 25: The Chemistry of Thioesters, Phosphate Esters, and Phosphate Anhydrides25 Questions
Exam 26: The Chemistry of the Aromatic Heterocycles and Nucleic Acids25 Questions
Exam 27: Amino Acids, Peptides, and Proteins25 Questions
Exam 28: Pericyclic Reactions25 Questions
Select questions type
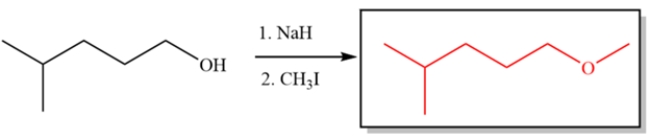
Provide the major organic product for the reaction sequence:
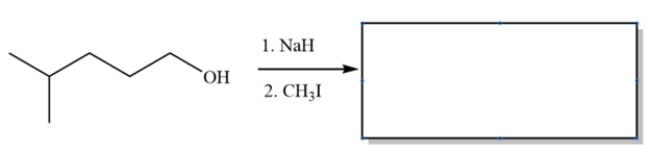
Free
(Essay)
4.7/5  (39)
(39)
Correct Answer:
This is an example of the Williamson ether synthesis. The first step deprotonates the alcohol to form an alkoxide. The second step is simply an SN2 substitution.
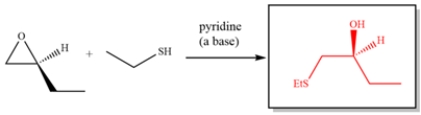
Complete the reaction:
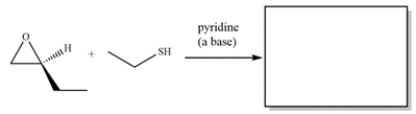
Free
(Essay)
4.9/5  (35)
(35)
Correct Answer:
Under basic or neutral conditions, a nucleophile will add to the less substituted epoxide carbon.
Propose a multistep synthesis for the epoxide (which will be prepared as the racemate) from 1-butyne, benzyl bromide, and any other reagents. As usual, show the reagents and the major organic product for each step.
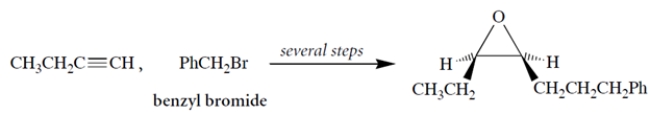
Free
(Essay)
4.9/5  (31)
(31)
Correct Answer:
There are many variations on the synthesis (see notes below the synthesis).
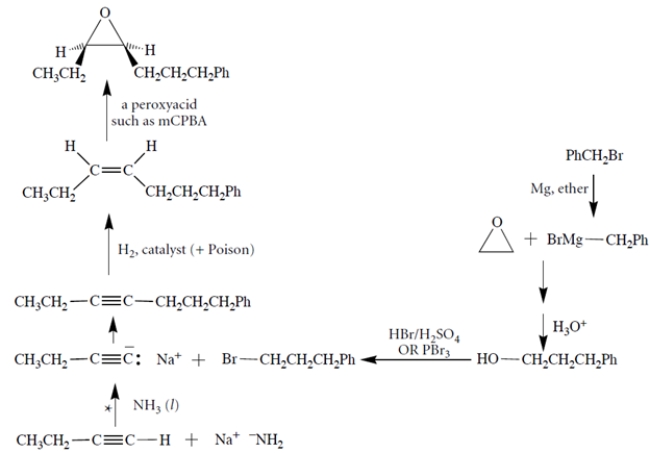 At (*) several variations are possible. For example, one could let benzyl bromide react with the conjugate base of acetylene, and then elaborate that into the alcohol via hydrogenation with a poisoned catalyst and hydroboration/oxidation. Or one could treat the alkene with HBr/peroxides.
At (*) several variations are possible. For example, one could let benzyl bromide react with the conjugate base of acetylene, and then elaborate that into the alcohol via hydrogenation with a poisoned catalyst and hydroboration/oxidation. Or one could treat the alkene with HBr/peroxides.
Compound A (C5H11BrO), when dissolved in ethanol containing one equivalent of NaOH, forms a product B. Identify compound A and give a two-step curved-arrow mechanism for its conversion into B. In each step of the curved-arrow mechanism identify the Brønsted acids (BA), Brønsted bases (BB), nucleophiles (N), electrophiles (E), and leaving groups (LG).
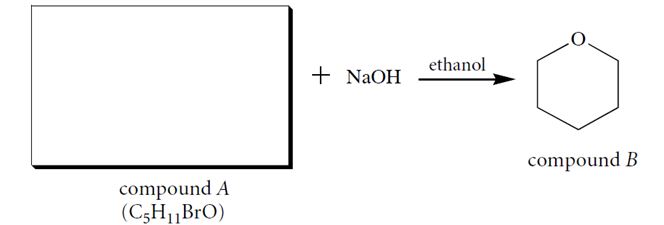
(Essay)
4.9/5  (35)
(35)
Complete the reaction by giving the major organic product. The product formed is a racemate-give the stereochemistry of either enantiomer.
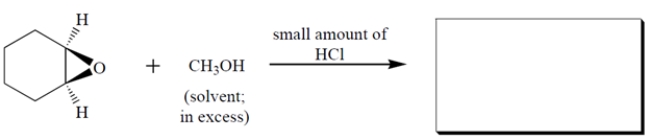
(Essay)
4.9/5  (34)
(34)
Using curved-arrow notation, propose a mechanism for the transformation that takes into account the stereochemistry of the product. (Hint: Note that two inversions of stereochemistry = one retention.)
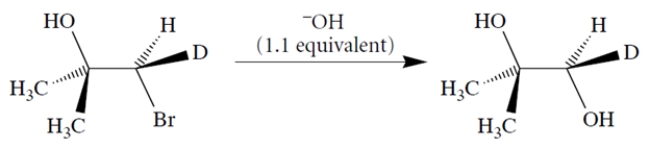
(Essay)
4.9/5  (38)
(38)
The reaction fails to generate the indicated ether. Explain why and what product is generated instead.
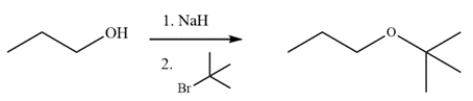
(Essay)
4.8/5  (42)
(42)
Give the structure of the nucleophile that would react with benzyl chloride (PhCH2Cl) to give the sulfonium salt:
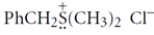
(Short Answer)
4.8/5  (30)
(30)
cis-3-Hexene is treated with OsO4 (osmium tetroxide), then water. Which one statement is true about the product?
(Multiple Choice)
4.9/5  (34)
(34)
Select the one true statement about biological reactions involving S-adenosylmethionine (SAM).
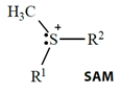
(Multiple Choice)
4.7/5  (31)
(31)
Complete the missing starting material. Be sure to clearly show stereochemistry.
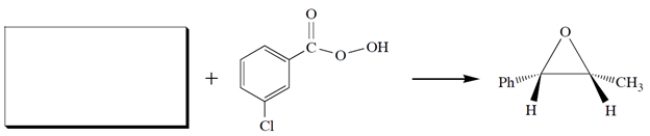
(Essay)
4.9/5  (32)
(32)
Propose a synthetic scheme to carry out the transformation. Show the product of each step.
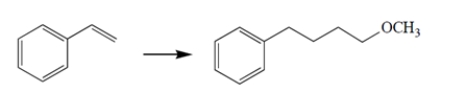
(Essay)
4.8/5  (40)
(40)
In the SN2 reaction, sulfur is the "leaving atom" in the leaving group. The products are a single sulfide (R-S-R´) and a single ether.
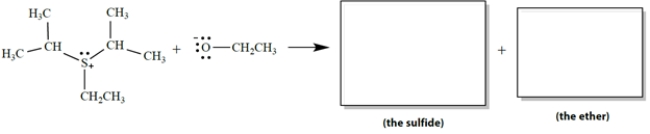 a. Draw the structures of the products in the appropriate boxes.
b. On the reaction, draw the curved-arrow notation for this reaction.
a. Draw the structures of the products in the appropriate boxes.
b. On the reaction, draw the curved-arrow notation for this reaction.
(Essay)
4.9/5  (31)
(31)
Provide the missing product and the curved-arrow notation for this reaction.
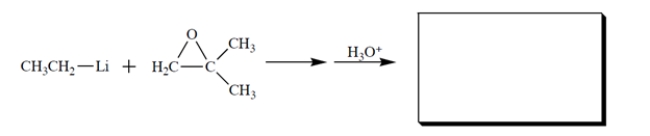
(Essay)
4.9/5  (36)
(36)
Complete the reaction sequence by providing the structures of the missing compounds.
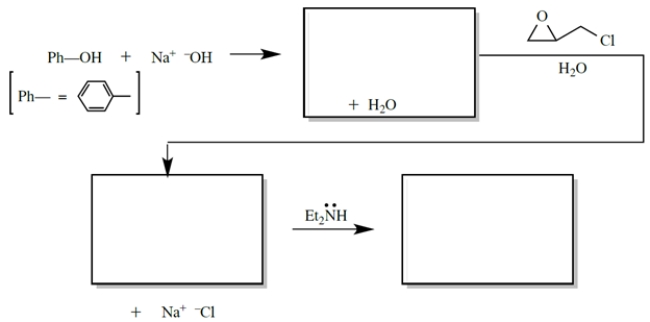
(Essay)
4.7/5  (38)
(38)
Provide the missing product. Clearly show stereochemistry of the product.
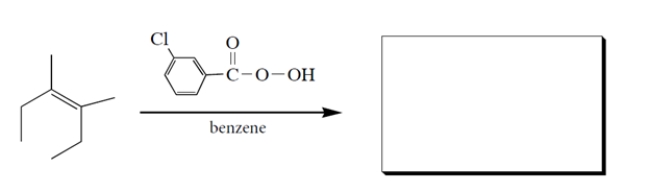
(Essay)
4.7/5  (35)
(35)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)