Deck 12: Peptides, Proteins, and Enzymes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/86
Play
Full screen (f)
Deck 12: Peptides, Proteins, and Enzymes
1
Which is an -amino acid?
A)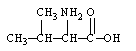
B)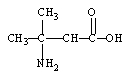
C)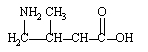
D)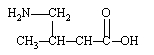
A)
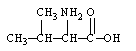
B)

C)
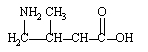
D)
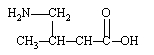
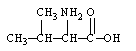
2
At physiological pH, most amino acids carry both a 1+ and 1- charge. This form is known as a ___.
A) zwitterion
B) anion
C) cation
D) isoelectric ion
A) zwitterion
B) anion
C) cation
D) isoelectric ion
zwitterion
3
Shown here is the amino acid glycine at a pH of ___. 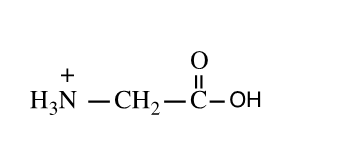
A) 1
B) 7
C) 14
D) both 1 and 14
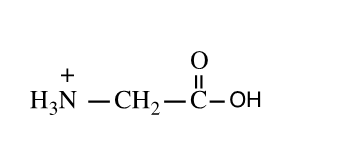
A) 1
B) 7
C) 14
D) both 1 and 14
1
4
Stereoisomers exist for most of the amino acids found in nature. Which statement below is correct?
A) Both the D-amino acids and the L- amino acids are used by living things.
B) Only the L-amino acids are incorporated in proteins.
C) A common conversion is from the D-amino acid to the L-amino acid.
D) L-amino acids are toxic.
A) Both the D-amino acids and the L- amino acids are used by living things.
B) Only the L-amino acids are incorporated in proteins.
C) A common conversion is from the D-amino acid to the L-amino acid.
D) L-amino acids are toxic.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
5
If an amino acid is in a neutral solution, the form the carboxyl group takes is ___.
A) -CO2-
B) -CO2H+
C) -CO2-
D) -CO2H
A) -CO2-
B) -CO2H+
C) -CO2-
D) -CO2H

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
6
The side chain of the amino acid phenylalanine is shown here.
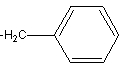 The side chain is classified as ___.
The side chain is classified as ___.
A) nonpolar
B) polar-acidic
C) polar-basic
D) polar-neutral
 The side chain is classified as ___.
The side chain is classified as ___.A) nonpolar
B) polar-acidic
C) polar-basic
D) polar-neutral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
7
The side chain of the amino acid tyrosine is shown here.
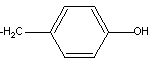 The side chain is classified as ___.
The side chain is classified as ___.
A) nonpolar
B) polar-acidic
C) polar-basic
D) polar-neutral
 The side chain is classified as ___.
The side chain is classified as ___.A) nonpolar
B) polar-acidic
C) polar-basic
D) polar-neutral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
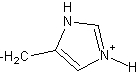
8
The side chain of the amino acid histidine is shown here.
The side chain is classified as ___.
A) nonpolar
B) polar-acidic
C) polar-basic
D) polar-neutral
The side chain is classified as ___.

A) nonpolar
B) polar-acidic
C) polar-basic
D) polar-neutral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
9
The structure of the amino acid glutamine is shown below. Glutamine can be classified as 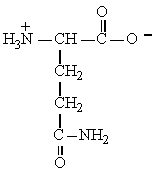
A) polar-neutral
B) polar -acidic
C) polar basic
D) nonpolar

A) polar-neutral
B) polar -acidic
C) polar basic
D) nonpolar

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
10
Shown here is the amino acid glycine at a pH of ___. 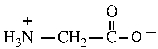
A) 1
B) 7
C) 14
D) both 1 and 14
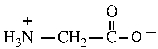
A) 1
B) 7
C) 14
D) both 1 and 14

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
11
The structures of the amino acids proline and glycine are shown below. Which of the following statements is true? 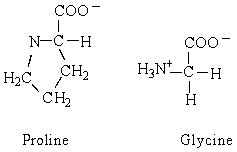
A) glycine is an -amino acid, but proline is not
B) both proline and glycine are chiral molecules
C) glycine is achiral and proline is chiral
D) proline exists as four enantiomers

A) glycine is an -amino acid, but proline is not
B) both proline and glycine are chiral molecules
C) glycine is achiral and proline is chiral
D) proline exists as four enantiomers

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
12
Shown here is the amino acid glycine at a pH of ___. 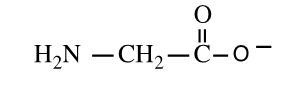
A) 1
B) 7
C) 14
D) both 1 and 14
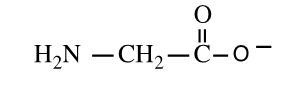
A) 1
B) 7
C) 14
D) both 1 and 14

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
13
Aspartic acid is classified as a polar-acidic amino acid due to 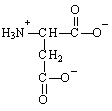
A) the -CH group to which the amide group is attached.
B) the -CH2 group in the middle of the side chain.
C) the amide group located on a side chain.
D) the carboxyl group located on the side chain.

A) the -CH group to which the amide group is attached.
B) the -CH2 group in the middle of the side chain.
C) the amide group located on a side chain.
D) the carboxyl group located on the side chain.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
14
What form does isoleucine take at pH 14?
A)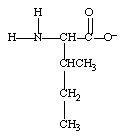
B)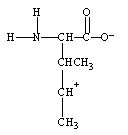
C)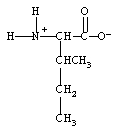
D)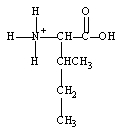
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
15
The peptide bond that connects amino acids in proteins is actually an ___ linkage.
A) amine
B) amide
C) ether
D) ester
A) amine
B) amide
C) ether
D) ester

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
16
The term commonly used for a chain of amino acids 100 units long is ___.
A) peptide
B) oligopeptide
C) polypeptide
D) centapeptide
A) peptide
B) oligopeptide
C) polypeptide
D) centapeptide

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
17
Which reaction is capable of breaking polypeptides into their component amino acids?
A) hydrogenation (adding hydrogen)
B) hydrolysis (adding water)
C) dehydrogenation (removing hydrogen)
D) dehydration (removing water)
A) hydrogenation (adding hydrogen)
B) hydrolysis (adding water)
C) dehydrogenation (removing hydrogen)
D) dehydration (removing water)

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
18
Leucine-enkephalin, a pentapeptide, is a naturally occurring pain reliever found in the brain. How many peptide bonds are there in leucine-enkephalin?
A) 5
B) 4
C) 6
D) 3
A) 5
B) 4
C) 6
D) 3

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
19
A tetrapeptide is given as: Ser-Lys-Ala-Pro. Which of the following statements is true?
A) The serine provided a nitrogen for the peptide bond.
B) The N-terminus is serine (Ser).
C) There are four peptide bonds because there are four amino acids.
D) The listing should be in alphabetical order.
A) The serine provided a nitrogen for the peptide bond.
B) The N-terminus is serine (Ser).
C) There are four peptide bonds because there are four amino acids.
D) The listing should be in alphabetical order.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
20
The form of dipeptide aspatylserine (Asp-Ser) obtained from two amino acids, aspartic acid and serine, whose structures are shown here, is 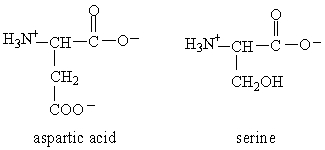
A)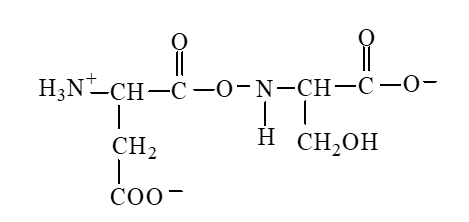
B)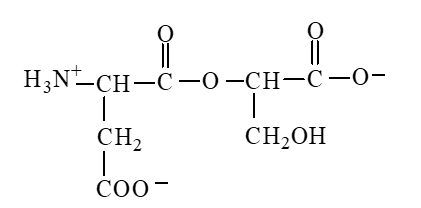
C)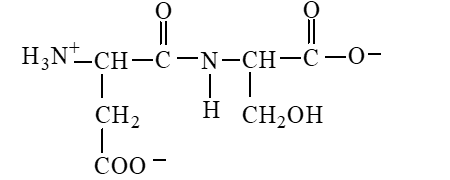
D)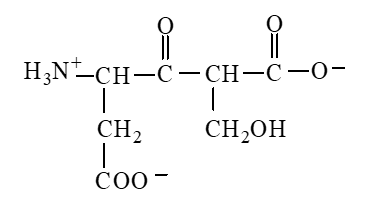

A)
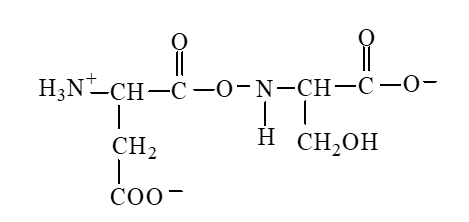
B)

C)
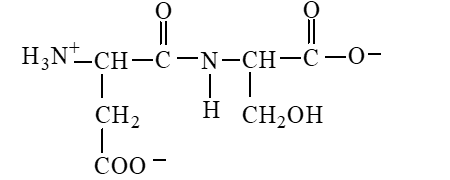
D)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
21
Where is the peptide bond located in this dipeptide?
A) the CO-NH joining serine and the valine
B) the doubly bonded oxygen just left of center between the amino acids
C) the CH-CO on the top of the serine going to the right
D) the NH-CH between the serine and valine

A) the CO-NH joining serine and the valine
B) the doubly bonded oxygen just left of center between the amino acids
C) the CH-CO on the top of the serine going to the right
D) the NH-CH between the serine and valine

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
22
What is the net charge on the oligopeptide in the figure shown here at pH 1? 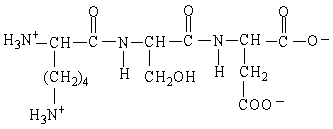
A) 3+
B) 1+
C) 2+
D) 0

A) 3+
B) 1+
C) 2+
D) 0

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
23
The structure of the dipeptide, Phe-Gly, in a solution of pH 14 is ___.
A)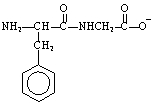
B)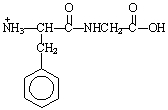
C)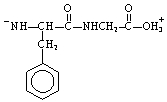
D)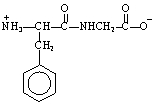
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
24
A biologically active protein referred to as a simple protein contains
A) very few hydrogen bonds.
B) only one heme group.
C) less than three different amino acids.
D) no prosthetic groups.
A) very few hydrogen bonds.
B) only one heme group.
C) less than three different amino acids.
D) no prosthetic groups.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
25
The element found in the center of the heme prosthetic group is ___.
A) iron
B) sulfur
C) nitrogen
D) carbon
A) iron
B) sulfur
C) nitrogen
D) carbon

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
26
Which interactions are involved in establishing and holding the three-dimensional structure of a protein?
A) salt bridge and disulfide bonds
B) hydrogen bonds
C) hydrophobic effects
D) all of these choices
A) salt bridge and disulfide bonds
B) hydrogen bonds
C) hydrophobic effects
D) all of these choices

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
27
Proteins tend to assume a specific overall three-dimensional shape. This is referred to as the protein's ___.
A) primary structure
B) secondary structure
C) tertiary structure
D) quaternary structure
A) primary structure
B) secondary structure
C) tertiary structure
D) quaternary structure

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
28
A contributing factor to the tertiary structure of a protein is a covalent bond between two atoms of ___.
A) sulfur
B) hydrogen
C) carbon
D) nitrogen
A) sulfur
B) hydrogen
C) carbon
D) nitrogen

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following sentences is true?
A) Rearranging the amino acid residues in a peptide or protein changes its function.
B) In an -helix form, portions of polypeptide chain line up side by side with hydrogen bonds holding neighboring strands of sheet together.
C) The order of amino acid residues in a protein is referred to as its tertiary structure.
D) The overall three-dimensional shape of a protein is referred to as its primary structure.
A) Rearranging the amino acid residues in a peptide or protein changes its function.
B) In an -helix form, portions of polypeptide chain line up side by side with hydrogen bonds holding neighboring strands of sheet together.
C) The order of amino acid residues in a protein is referred to as its tertiary structure.
D) The overall three-dimensional shape of a protein is referred to as its primary structure.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
30
The overall three-dimensional shape of a protein is determined by its ___ and is known as the ___ structure of a protein.
A) amino acid residues/primary
B) amino acid residues/tertiary
C) isoelectric point/secondary
D) hydrogen bonding/secondary
A) amino acid residues/primary
B) amino acid residues/tertiary
C) isoelectric point/secondary
D) hydrogen bonding/secondary

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following do not contribute to the overall stability of a tertiary structure of proteins?
A) salt bridges
B) hydrogen bonding
C) hydrophobic effect
D) hormones
A) salt bridges
B) hydrogen bonding
C) hydrophobic effect
D) hormones

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
32
Globular proteins in water typically assume their specific shapes because
A) they are water-soluble due to the polar points located along the length of the protein molecules.
B) the nonpolar portion of the molecule is inside and the polar portion is outside, as stated by the folding rule.
C) they can assume the -sheet form, which stretches along the surface of the solution.
D) all of the polar side chains in the molecular structure fold inward to be protected from the effects of the polarity of water.
A) they are water-soluble due to the polar points located along the length of the protein molecules.
B) the nonpolar portion of the molecule is inside and the polar portion is outside, as stated by the folding rule.
C) they can assume the -sheet form, which stretches along the surface of the solution.
D) all of the polar side chains in the molecular structure fold inward to be protected from the effects of the polarity of water.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
33
The hydrophobic portions of a protein tend to
A) form chemical bonds between them so that the structure of the protein is rigid.
B) be attracted to each other by means of strong interatomic forces.
C) be strongly attracted to the water in the surroundings, pulling portions of the molecule away from its center.
D) fold into the interior of the three-dimensional shape away from water.
A) form chemical bonds between them so that the structure of the protein is rigid.
B) be attracted to each other by means of strong interatomic forces.
C) be strongly attracted to the water in the surroundings, pulling portions of the molecule away from its center.
D) fold into the interior of the three-dimensional shape away from water.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
34
In which of the following levels of protein structure can hydrogen bonding NOT play a role?
A) primary
B) secondary
C) tertiary
D) quaternary
A) primary
B) secondary
C) tertiary
D) quaternary

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
35
Proteins can contain prosthetic groups, which are
A) polypeptide chains that contain only one kind of amino acid.
B) polypeptide chains that come off the main chain to form a branch.
C) groups containing nonpeptide components.
D) groups of amino acids that form accessory structures to the protein.
A) polypeptide chains that contain only one kind of amino acid.
B) polypeptide chains that come off the main chain to form a branch.
C) groups containing nonpeptide components.
D) groups of amino acids that form accessory structures to the protein.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
36
What kind of noncovalent interaction occurs between the -NH3+ ending of one side chain of an amino acid with the -COO- ending of sidechain of another amino acid in tertiary structure of a protein?
A) salt bridge
B) hydrogen bonding
C) covalent disulfide bond
D) hydrophobic effect
A) salt bridge
B) hydrogen bonding
C) covalent disulfide bond
D) hydrophobic effect

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following substances recognized by immune system evoke production of one or more antibodies?
A) secondary proteins
B) antigens
C) globular proteins
D) glycolipids
A) secondary proteins
B) antigens
C) globular proteins
D) glycolipids

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
38
Which is a means of denaturing a protein?
A) changes in temperature
B) changes in pH
C) use of detergents or soaps
D) all of these choices
A) changes in temperature
B) changes in pH
C) use of detergents or soaps
D) all of these choices

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
39
The ___ is the location on the protein molecule where enzyme catalyzed reactions take place.
A) substrate
B) zymogen
C) active site
D) coenzyme
A) substrate
B) zymogen
C) active site
D) coenzyme

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
40
An enzyme displays ___ when the enzyme accepts only one specific substrate.
A) absolute specificity
B) relative specificity
C) stereospecificity
D) no specificity
A) absolute specificity
B) relative specificity
C) stereospecificity
D) no specificity

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
41
Catalysts, including enzymes, have the role of
A) changing a nonspontaneous chemical reaction to spontaneous.
B) lowering the activation energy of a reaction.
C) heating up the reactants to make the reaction progress faster.
D) changing the pH to a favorable pH for the reaction to progress.
A) changing a nonspontaneous chemical reaction to spontaneous.
B) lowering the activation energy of a reaction.
C) heating up the reactants to make the reaction progress faster.
D) changing the pH to a favorable pH for the reaction to progress.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
42
The enzyme known as alcohol dehydrogenase is capable of oxidizing ethanol, methanol and other hydroxyl group containing organic compounds into their corresponding aldehydes. Such an enzyme with ___ catalyzes the reaction of structurally related substances.
A) absolute specificity
B) relative specificity
C) stereospecificity
D) no specificity
A) absolute specificity
B) relative specificity
C) stereospecificity
D) no specificity

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
43
Organic compounds that are not polypeptides but are necessary for enzymes to properly function are called ___.
A) substrates
B) zymogens
C) active sites
D) coenzymes
A) substrates
B) zymogens
C) active sites
D) coenzymes

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
44
A change in pH usually produces a change in enzyme activity because
A) pH of the environment changes the conformation of the enzyme.
B) enzyme activity increases at extreme pH values (either acidic or basic).
C) enzyme activity is usually least active below its optimum pH.
D) enzyme activity is most active above its optimum temperature.
A) pH of the environment changes the conformation of the enzyme.
B) enzyme activity increases at extreme pH values (either acidic or basic).
C) enzyme activity is usually least active below its optimum pH.
D) enzyme activity is most active above its optimum temperature.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
45
Many of the chemical reactions of living things involve enzymes, which are
A) proteins that are in the form of parallel sheets.
B) proteins that catalyze reactions.
C) small DNA molecules.
D) denatured proteins.
A) proteins that are in the form of parallel sheets.
B) proteins that catalyze reactions.
C) small DNA molecules.
D) denatured proteins.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
46
A certain enzyme's name is glucose-6-phosphorylase. This enzyme most likely catalyzes a reaction that
A) converts glucose into six phosphorous atoms.
B) synthesizes six glucose molecules.
C) joins six glucose molecules to a phosphate.
D) places a phosphate on the sixth carbon of glucose.
A) converts glucose into six phosphorous atoms.
B) synthesizes six glucose molecules.
C) joins six glucose molecules to a phosphate.
D) places a phosphate on the sixth carbon of glucose.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
47
The enzyme hexokinase catalyzes the transfer of a phosphate group from ATP to carbon 6 of D-glucose and other D-hexoses. L-hexoses are not substrates to the enzyme.
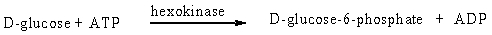 Hexokinase displays
Hexokinase displays
A) relative specificity
B) stereospecificity
C) absolute specificity
D) both relative specificity and stereospecificity
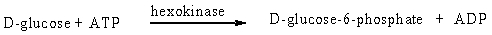 Hexokinase displays
Hexokinase displaysA) relative specificity
B) stereospecificity
C) absolute specificity
D) both relative specificity and stereospecificity

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
48
___ are inactive enzyme precursors that are synthesized, stored and may be activated where needed.
A) Substrates
B) Zymogens
C) Active sites
D) Coenzymes
A) Substrates
B) Zymogens
C) Active sites
D) Coenzymes

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
49
An inhibitor is a substance that affects an enzyme by
A) reducing the ability to act as a catalyst.
B) removing the prosthetic group from an enzyme.
C) reacting with the enzyme so there is less effect of the chemical reaction's surroundings on the rate of reaction.
D) stopping the separation of the enzyme from the product keeping the reaction from going to completion.
A) reducing the ability to act as a catalyst.
B) removing the prosthetic group from an enzyme.
C) reacting with the enzyme so there is less effect of the chemical reaction's surroundings on the rate of reaction.
D) stopping the separation of the enzyme from the product keeping the reaction from going to completion.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
50
The function of feedback inhibition is to
A) affect the polarity of a substrate so that it will not be picked up by an enzyme.
B) provide a stimulus to the enzyme that makes it active during a reaction.
C) provide a way of keeping the wrong substrate from coming in contact with an enzyme.
D) control an enzyme reaction so that there isn't an overproduction of the product.
A) affect the polarity of a substrate so that it will not be picked up by an enzyme.
B) provide a stimulus to the enzyme that makes it active during a reaction.
C) provide a way of keeping the wrong substrate from coming in contact with an enzyme.
D) control an enzyme reaction so that there isn't an overproduction of the product.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
51
An allosteric enzyme differs from a Michaelis-Menten enzyme because the allosteric enzyme
A) has a more active binding site that is more polar and will hold the substrate longer.
B) displays cooperativity in which the binding of a substrate to the active site on one subunit affects the binding of substrate at other subunits.
C) operates to change reaction rates of all of the stereoisomers of a compound, not just one.
D) will catalyze all the reactions of a substance produced in a series of steps.
A) has a more active binding site that is more polar and will hold the substrate longer.
B) displays cooperativity in which the binding of a substrate to the active site on one subunit affects the binding of substrate at other subunits.
C) operates to change reaction rates of all of the stereoisomers of a compound, not just one.
D) will catalyze all the reactions of a substance produced in a series of steps.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
52
A characteristic of a Michaelis-Menten enzyme is that it
A) inhibits the formation of a complex under the reaction conditions.
B) forms an enzyme-substrate complex that is stable.
C) forms an enzyme-substrate complex after which the product and the enzyme separate.
D) is a system specific to the hydrolysis of complex carbohydrates.
A) inhibits the formation of a complex under the reaction conditions.
B) forms an enzyme-substrate complex that is stable.
C) forms an enzyme-substrate complex after which the product and the enzyme separate.
D) is a system specific to the hydrolysis of complex carbohydrates.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
53
When a patient has accidentally ingested methanol, a physician can order the use of ethanol to overwhelm the alcohol dehydrogenase enzyme. This prevents the liver from producing deadly formaldehyde from the methanol. This is an example of _____.
A) covalent modification
B) feedback inhibition
C) competitive inhibition
D) protein denaturation
A) covalent modification
B) feedback inhibition
C) competitive inhibition
D) protein denaturation

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
54
Alanine is the only -amino acid that does not have a chiral carbon.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
55
Because amino acids contain polar covalent bonds, they are hydrophilic and will dissolve water.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
56
The net charge of peptides and proteins affects their solubility.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
57
Hydrogen bonding plays a role in the primary structure of protein.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
58
Fibrous proteins, because of their long fibers, tend to be soluble in water.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
59
Protein molecules with the appropriate structure to be biologically active are native molecules.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
60
A characteristic that distinguishes between secondary and tertiary structures is the fact that hydrogen bonding in all secondary structures are between backbone -C=O and H-N- groups.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
61
Proteins can be denatured by changes in temperature or pH.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
62
Cofactors for some enzymes are not considered prosthetic groups because they are loosely held during the course of reaction.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
63
Enzymes that display stereospecificity are those that will catalyze the reactions of both the L-form and the D-form of a carbohydrate.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
64
Noncompetitive inhibitors resemble the substrate and bond to the active site of an enzyme.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
65
___ is the pH at which an amino acid solution has no net charge because it has equal positive and negative charges.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
66
___ are composed of more than 10 amino acid residues.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
67
The amide bond between two amino acids is referred to as a ___ bond.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
68
The oligopeptides have from 2 to ___ amino acid units in their molecules.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
69
As in α-helix, and the β-sheet are held together by ___ between amide-N-H and C=O groups.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
70
The ___ of a protein largely determines its secondary and tertiary structures.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
71
Proteins with quaternary structures have structures involving two or more ___ that are independent of each other.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
72
Tertiary structure for some proteins sometimes include nonpeptide groups called ___, and proteins that require a such groups for biological activity are called ___ proteins.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
73
In a treatment called ___, a monoclonal antibody is combined with a radioisotope.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
74
If a biologically active protein is sufficiently changed in its conformation so that it no longer is biologically active, that protein has been ___.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
75
Enzymes that only catalyze the reaction of a particular stereoisomer are referred to as ___.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
76
A ___ inhibitor is a substance that attaches to an enzyme at sites other than the active sites causing the enzyme to lose its ability to bind with the appropriate substrate.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
77
Enzymes that are composed of more than one peptide chain and for which binding of a substrate to one active site affects the bonding of the substrate to other active sites are referred to as a(n) ___ enzyme(s).

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
78
Given the following amino acids and their R groups:
glycine (R group is -H) - aspartic acid (R group is -CH2COO-)
cysteine (R group is -CH2SH) - lysine (R group is -(CH2)4NH3+)
Sketch a generic amino acid.
glycine (R group is -H) - aspartic acid (R group is -CH2COO-)
cysteine (R group is -CH2SH) - lysine (R group is -(CH2)4NH3+)
Sketch a generic amino acid.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
79
Given the following amino acids and their R groups:
glycine (R group is -H) - aspartic acid (R group is -CH2COO-)
cysteine (R group is -CH2SH) - lysine (R group is -(CH2)4NH3+)
Which amino acid is not optically active?
glycine (R group is -H) - aspartic acid (R group is -CH2COO-)
cysteine (R group is -CH2SH) - lysine (R group is -(CH2)4NH3+)
Which amino acid is not optically active?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
80
Given the following amino acids and their R groups:
glycine (R group is -H) - aspartic acid (R group is -CH2COO-)
cysteine (R group is -CH2SH) - lysine (R group is -(CH2)4NH3+)
Sketch cysteine.
glycine (R group is -H) - aspartic acid (R group is -CH2COO-)
cysteine (R group is -CH2SH) - lysine (R group is -(CH2)4NH3+)
Sketch cysteine.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck


