Deck 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds
1
Which of the following statements is true about alkanes?
A) alkanes contain polar bonds
B) alkanes are attracted to one another by London forces
C) alkanes are polar molecules
D) alkanes are unsaturated hydrocarbons
A) alkanes contain polar bonds
B) alkanes are attracted to one another by London forces
C) alkanes are polar molecules
D) alkanes are unsaturated hydrocarbons
alkanes are attracted to one another by London forces
2
Hydocarbons are organic compounds that
A) contain carbon and hydrogen atoms only.
B) are soluble in water.
C) are polar molecules.
D) contain carbon, hydrogen, and halogen atoms.
A) contain carbon and hydrogen atoms only.
B) are soluble in water.
C) are polar molecules.
D) contain carbon, hydrogen, and halogen atoms.
contain carbon and hydrogen atoms only.
3
Which of the following molecules is an alkane?
A)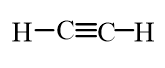
B)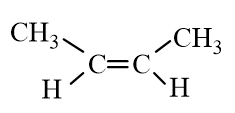
C)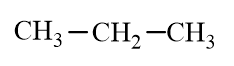
D)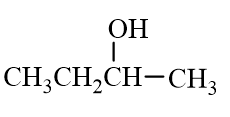
A)
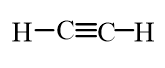
B)

C)
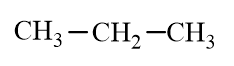
D)
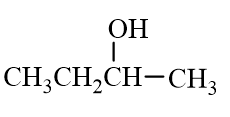
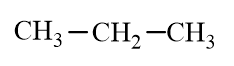
4
Give the correct IUPAC name for the following molecule: 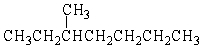
A) 2-Ethylpentane
B) 3-Methylhexane
C) 3-Methylcycloheptane
D) 3-Methylheptane
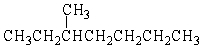
A) 2-Ethylpentane
B) 3-Methylhexane
C) 3-Methylcycloheptane
D) 3-Methylheptane

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
Give the correct IUPAC name for the following molecule: 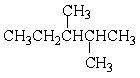
A) 3, 4-Dimethylpentane
B) 2,3-Dimethyl heptane
C) 2,3-Dimethylpentane
D) 1,1,2-Trimethylpentane
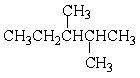
A) 3, 4-Dimethylpentane
B) 2,3-Dimethyl heptane
C) 2,3-Dimethylpentane
D) 1,1,2-Trimethylpentane

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is true about nonane?
A) It is a polar molecule.
B) It dissolves in water.
C) It is a liquid at room temperature.
D) It contains one double bond.
A) It is a polar molecule.
B) It dissolves in water.
C) It is a liquid at room temperature.
D) It contains one double bond.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following structures is a constitutional isomer of 2-Methylheptane?
A)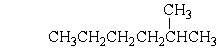
B)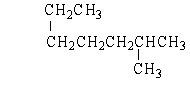
C)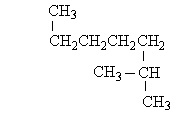
D)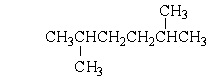
A)
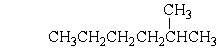
B)
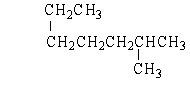
C)
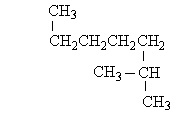
D)
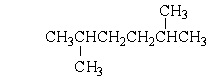

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
The name of the following compound is 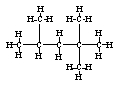
A) 2,2,4-trimethylpentane.
B) 1,1,1,3-tetramethylbutane.
C) 1,1,1,3,3-pentamethylpropane.
D) Both 1,1,1,3-tetramethylbutane and 1,1,1,3,3-pentamethylpropane are correct names.

A) 2,2,4-trimethylpentane.
B) 1,1,1,3-tetramethylbutane.
C) 1,1,1,3,3-pentamethylpropane.
D) Both 1,1,1,3-tetramethylbutane and 1,1,1,3,3-pentamethylpropane are correct names.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following pairs of compounds represent constitutional isomers?
A) 2-methylpropane and Pentane
B) 2,2-dimethylbutane and 3-methylpentane
C) 2,2-dimethylpropane and 2-methylpentane
D) 2-methylbutane and 2-methylpropane
A) 2-methylpropane and Pentane
B) 2,2-dimethylbutane and 3-methylpentane
C) 2,2-dimethylpropane and 2-methylpentane
D) 2-methylbutane and 2-methylpropane

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
The following is an example of what type of reaction? 
A) Hydration
B) Combustion
C) Hydrogeneation
D) Halogenation

A) Hydration
B) Combustion
C) Hydrogeneation
D) Halogenation

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
The correct IUPAC name for the molecule below is ___. 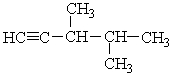
A) 1-pentene
B) 2,3-dimethyl-1-pentyne
C) 2,3,3-trimethyl-1-butyne
D) 2,3-dimethyl-1-heptene

A) 1-pentene
B) 2,3-dimethyl-1-pentyne
C) 2,3,3-trimethyl-1-butyne
D) 2,3-dimethyl-1-heptene

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following molecules represent trans- 2-Hexene?
A)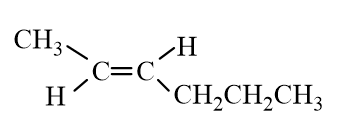
B)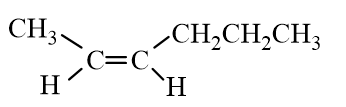
C)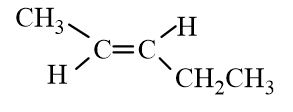
D)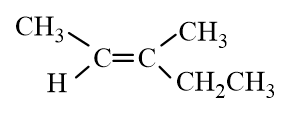
A)
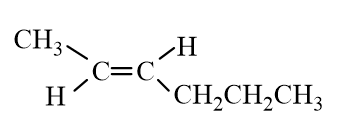
B)

C)

D)


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
What is the major organic product of the following reaction? 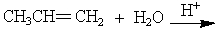
A) 1-propanol
B) 2-propanol
C) propanal
D) propanone
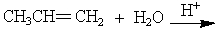
A) 1-propanol
B) 2-propanol
C) propanal
D) propanone

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
Provide IUPAC name for the following molecule. 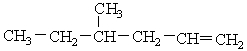
A) 3-methyl-5-Hexene
B) 4-ethyl-1-pentene
C) 3-methyl-5-heptene
D) 4-methyl-1-hexene
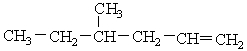
A) 3-methyl-5-Hexene
B) 4-ethyl-1-pentene
C) 3-methyl-5-heptene
D) 4-methyl-1-hexene

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
What is the major product of the reaction below? 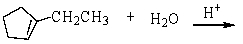
A)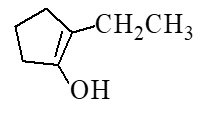
B)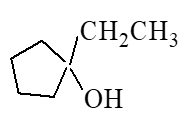
C)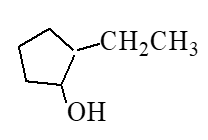
D)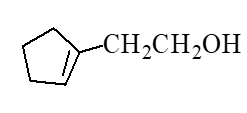
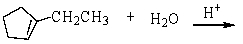
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following can participate in hydrogen bonding?
A) alcohols
B) carboxylic acids
C) primary amines
D) all of these choices
A) alcohols
B) carboxylic acids
C) primary amines
D) all of these choices

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
Rank butane (CH3CH2CH2CH3), butanoic acid (CH3CH2CH2COOH), and pentane (CH3CH2CH2CH2CH3) in order of increasing boiling point. (From lowest to highest.)
A) butane < butanoic acid < pentane
B) butane < pentane < butanoic acid
C) butanoic acid < pentane < butane
D) pentane < butanoic acid < butane
A) butane < butanoic acid < pentane
B) butane < pentane < butanoic acid
C) butanoic acid < pentane < butane
D) pentane < butanoic acid < butane

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
Carboxylic acids, when compared with other molecules of similar size,
A) have relatively lower melting points.
B) have relatively higher boiling points.
C) have a smaller number of hydrogen bonds.
D) have more nonpolar locations.
A) have relatively lower melting points.
B) have relatively higher boiling points.
C) have a smaller number of hydrogen bonds.
D) have more nonpolar locations.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
Give the IUPAC name of the following carboxylic acid? 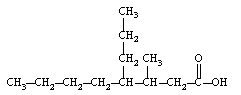
A) 4-propylnanoic acid
B) 3-octyl-butanoic acid
C) 4-butyl-3-methylheptanoic acid
D) 3-methyl-4-propyloctanoic acid

A) 4-propylnanoic acid
B) 3-octyl-butanoic acid
C) 4-butyl-3-methylheptanoic acid
D) 3-methyl-4-propyloctanoic acid

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name of the molecule below? 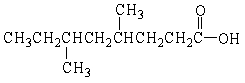
A) octanoic acid
B) 6-ethyl-4-methylhexanoic acid
C) 4,6-dimethyloctanoic acid
D) 3,5-dimethyloctanoic acid
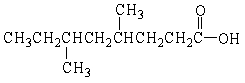
A) octanoic acid
B) 6-ethyl-4-methylhexanoic acid
C) 4,6-dimethyloctanoic acid
D) 3,5-dimethyloctanoic acid

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
Provide the IUPAC name for the compound below. 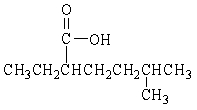
A) 2-methyl-5-heptanoic acid
B) 2-ethyl-5-methylhexanoic acid
C) 6-methyl-3-heptanoic acid
D) 6-methylheptanoic acid

A) 2-methyl-5-heptanoic acid
B) 2-ethyl-5-methylhexanoic acid
C) 6-methyl-3-heptanoic acid
D) 6-methylheptanoic acid

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
The molecule responsible for the hot taste of chili peppers is capsaicin. Capsaicin is a phenol because it has
A) a double bond.
B) an amide within its structure.
C) a trans stereochemistry at its double bond.
D) an -OH attached to a benzene ring.
A) a double bond.
B) an amide within its structure.
C) a trans stereochemistry at its double bond.
D) an -OH attached to a benzene ring.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
The carboxylic acids share which characteristic with the phenols?
A) They have relatively high boiling points.
B) They are capable of forming hydrogen bonds.
C) They are weak acids.
D) All of these answer choices are correct.
A) They have relatively high boiling points.
B) They are capable of forming hydrogen bonds.
C) They are weak acids.
D) All of these answer choices are correct.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
The IUPAC name of the molecule below is 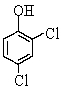
A) hydroxyl -2,4-dichlorocyclohexene
B) 3,4-dichlorophenol
C) 2,4-dichlorophenol
D) 1,3-dichlorophenol

A) hydroxyl -2,4-dichlorocyclohexene
B) 3,4-dichlorophenol
C) 2,4-dichlorophenol
D) 1,3-dichlorophenol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
Phenol and toluene (structures shown below) have similar molecular weights.
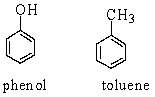 Which of the following statements accounts for the higher boiling point of phenol (182 C) than that of toluene (111 C)?
Which of the following statements accounts for the higher boiling point of phenol (182 C) than that of toluene (111 C)?
A) Toluene is more soluble in water than phenol.
B) Phenol molecules are primarily attracted to one another by London forces.
C) Toluene has greater hydrogen bonding interactions than phenol.
D) Phenol molecules are primarily attracted to one another by hydrogen bonds.
 Which of the following statements accounts for the higher boiling point of phenol (182 C) than that of toluene (111 C)?
Which of the following statements accounts for the higher boiling point of phenol (182 C) than that of toluene (111 C)?A) Toluene is more soluble in water than phenol.
B) Phenol molecules are primarily attracted to one another by London forces.
C) Toluene has greater hydrogen bonding interactions than phenol.
D) Phenol molecules are primarily attracted to one another by hydrogen bonds.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
The carboxylic acids with less than 11 carbons are likely to have
A) lower vapor pressure than the larger carboxylic acids.
B) lower water solubility than larger carboxylic acids.
C) lower boiling points than the larger carboxylic acids.
D) the same chemical and physical properties as those with 11 to 20 carbons.
A) lower vapor pressure than the larger carboxylic acids.
B) lower water solubility than larger carboxylic acids.
C) lower boiling points than the larger carboxylic acids.
D) the same chemical and physical properties as those with 11 to 20 carbons.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following produce acidic solutions in water?
A) phenols
B) 2º amines
C) carboxylic acids
D) both phenols and carboxylic acids
A) phenols
B) 2º amines
C) carboxylic acids
D) both phenols and carboxylic acids

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following lowers the pH when added to water?
A) ethyl alcohol
B) propanoic acid
C) methyl amine
D) methyl alcohol
A) ethyl alcohol
B) propanoic acid
C) methyl amine
D) methyl alcohol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
The chapter refers to physiological pH, which is approximately a
A) pH of 6.3.
B) pH of 7.
C) pH of 8.
D) pH of 10.
A) pH of 6.3.
B) pH of 7.
C) pH of 8.
D) pH of 10.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
Phenols are organic acids, as are carboxylic acids. The difference is that
A) the phenols have a Ka that is larger than the carboxylic acids.
B) the phenols are much stronger acids.
C) the carboxylic acids are significantly stronger.
D) the carboxylic acids are much larger than the phenols.
A) the phenols have a Ka that is larger than the carboxylic acids.
B) the phenols are much stronger acids.
C) the carboxylic acids are significantly stronger.
D) the carboxylic acids are much larger than the phenols.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
Both carboxylic acids and phenols react with strong bases to produce
A) the conjugate base of the carboxylic acid or phenol.
B) a stronger acid or phenol.
C) a complex with water.
D) gaseous products.
A) the conjugate base of the carboxylic acid or phenol.
B) a stronger acid or phenol.
C) a complex with water.
D) gaseous products.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
Provide the organic product formed when NaOH reacts with 3-bromophenol.
A) sodium 3-bromophenoxide
B) sodium phenoxide
C) 3-bromobenzoic acid
D) 3-bromobenzoate
A) sodium 3-bromophenoxide
B) sodium phenoxide
C) 3-bromobenzoic acid
D) 3-bromobenzoate

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
Given: benzene, phenol, and sodium phenoxide. Rank these in order of increasing water solubility. (From lowest to highest.)
A) benzene < phenol < sodium phenoxide
B) phenol < benzene < sodium phenoxide
C) benzene < sodium phenoxide < phenol
D) sodium phenoxide < phenol < benzene
A) benzene < phenol < sodium phenoxide
B) phenol < benzene < sodium phenoxide
C) benzene < sodium phenoxide < phenol
D) sodium phenoxide < phenol < benzene

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
The forces that hold one ester molecule to another are predominantly
A) hydrogen bonding.
B) dipole-dipole interactions and London forces.
C) ionic.
D) covalent.
A) hydrogen bonding.
B) dipole-dipole interactions and London forces.
C) ionic.
D) covalent.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
Carboxylic acids and alcohols can react under acidic conditions
A) to produce compounds that exist only under acidic conditions.
B) to produce esters with water being formed.
C) to produce phenols, if the appropriate hydrolyzing agents are present.
D) only if the carboxylic acid is an extremely strong acid.
A) to produce compounds that exist only under acidic conditions.
B) to produce esters with water being formed.
C) to produce phenols, if the appropriate hydrolyzing agents are present.
D) only if the carboxylic acid is an extremely strong acid.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
The organic product of the reaction between NaOH and 3-methylbutanoic acid is named
A) 3-methylbutane sodium.
B) 3-methylbutanoate.
C) methylbutanoate.
D) sodium 3-methylbutanoate.
A) 3-methylbutane sodium.
B) 3-methylbutanoate.
C) methylbutanoate.
D) sodium 3-methylbutanoate.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
The organic product formed when propanoic acid reacts with ethanol in the presence of an acid catalyst is called
A) ethyl propanoic acid.
B) ethylpropanoate.
C) propylethanoate.
D) propylethanoic acid.
A) ethyl propanoic acid.
B) ethylpropanoate.
C) propylethanoate.
D) propylethanoic acid.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
The reaction below is an example of what kind of reaction? 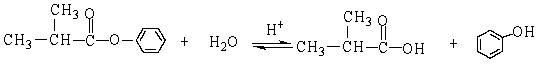
A) neutralization
B) hydrolysis
C) esterification
D) saponification

A) neutralization
B) hydrolysis
C) esterification
D) saponification

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
The missing product from the following reaction is 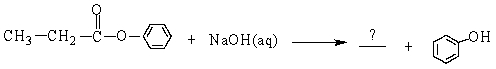
A)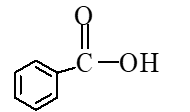
B)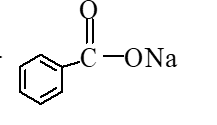
C)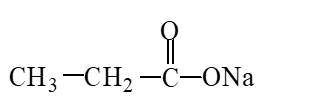
D)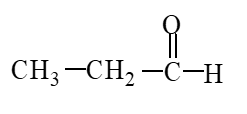
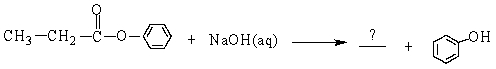
A)

B)

C)
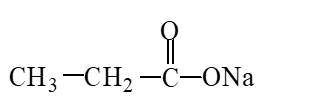
D)
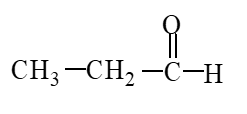

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
When the ester ethyl butanoate, CH3CH2CH2COOCH2CH3, is hydrolyzed under acidic conditions, which compounds are the products?
A) ethyl alcohol and butanoic acid
B) butanol and ethanoic (acetic acid)
C) ethyl alcohol and the butanoate ion
D) butanol and the ethanoate ion
A) ethyl alcohol and butanoic acid
B) butanol and ethanoic (acetic acid)
C) ethyl alcohol and the butanoate ion
D) butanol and the ethanoate ion

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
When the ester ethyl butanoate, CH3CH2CH2COOCH2CH3, is hydrolyzed under basic conditions, which compounds are the products?
A) ethyl alcohol and butanoic acid
B) butanol and ethanoic (acetic) acid
C) ethyl alcohol and the butanoate ion
D) butanol and the ethanoate ion
A) ethyl alcohol and butanoic acid
B) butanol and ethanoic (acetic) acid
C) ethyl alcohol and the butanoate ion
D) butanol and the ethanoate ion

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
Which raises the pH when added to water?
A) ethyl alcohol
B) propanoic acid
C) methyl amine
D) methyl alcohol
A) ethyl alcohol
B) propanoic acid
C) methyl amine
D) methyl alcohol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
The structure of amphetamine is shown.
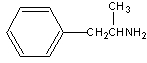 Amphetamine is a ___ amine.
Amphetamine is a ___ amine.
A) 1o
B) 2o
C) 3o
D) 4o
 Amphetamine is a ___ amine.
Amphetamine is a ___ amine.A) 1o
B) 2o
C) 3o
D) 4o

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
The amide containing anti-flea drug Lufenuron is hydrophobic, therefore it dissolves easily in ___.
A) fatty tissue
B) aqueous solutions
C) hair
D) water
A) fatty tissue
B) aqueous solutions
C) hair
D) water

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
To which class of compounds would a physiological stimulant most likely belong?
A) carboxylic acids
B) phenols
C) alcohols
D) amines
A) carboxylic acids
B) phenols
C) alcohols
D) amines

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
Which compound is not an amine?
A)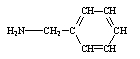
B)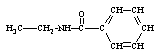
C)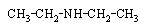
D)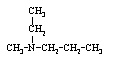
A)

B)
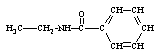
C)
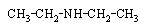
D)
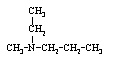

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
This compound is mescaline, a hallucinogen.
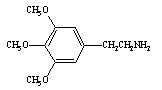 Which of the following statements is true?
Which of the following statements is true?
A) Mescaline is an amine.
B) Mescaline contains an alcohol group.
C) Mescaline is a carboxylic acid.
D) Mescaline is an amine and contains an alcohol group.
 Which of the following statements is true?
Which of the following statements is true?A) Mescaline is an amine.
B) Mescaline contains an alcohol group.
C) Mescaline is a carboxylic acid.
D) Mescaline is an amine and contains an alcohol group.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
What is the name of this compound? 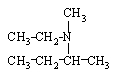
A) ethylmethyl-sec-butyl-ammonium
B) ethylmethylbutylammonium
C) N-ethyl-N-methyl-3-butamine
D) N-ethyl-N-methyl-2-butanamine

A) ethylmethyl-sec-butyl-ammonium
B) ethylmethylbutylammonium
C) N-ethyl-N-methyl-3-butamine
D) N-ethyl-N-methyl-2-butanamine

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
Given the following amines:
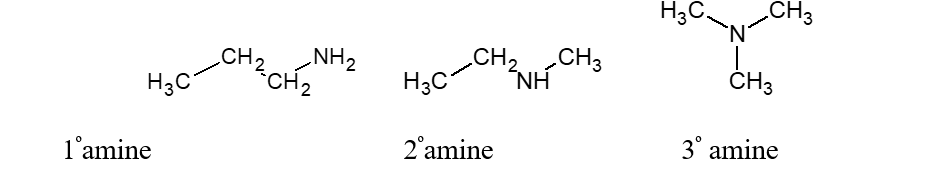 These three amines all have the same formula weight. Rank them in order of increasing boiling point. (From lowest to highest.)
These three amines all have the same formula weight. Rank them in order of increasing boiling point. (From lowest to highest.)
A) 1º < 2º < 3º
B) 2º < 1º < 3º
C) 3º < 2º < 1º
D) 3º < 1º < 2º
 These three amines all have the same formula weight. Rank them in order of increasing boiling point. (From lowest to highest.)
These three amines all have the same formula weight. Rank them in order of increasing boiling point. (From lowest to highest.)A) 1º < 2º < 3º
B) 2º < 1º < 3º
C) 3º < 2º < 1º
D) 3º < 1º < 2º

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
Provide the IUPAC name for the following organic compound. 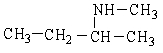
A) N-Methyl-2-butamine
B) methylbutanamine
C) dimethypropyllamine
D) butylmethanamine
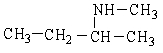
A) N-Methyl-2-butamine
B) methylbutanamine
C) dimethypropyllamine
D) butylmethanamine

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
Provide the IUPAC name for the following organic compound. 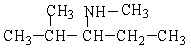
A) methylpentanamine
B) dimethylpentanamine
C) 2,N-Dimethyl-3-pentanamine
D) N-methyl-2-methylpentanamine
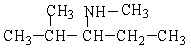
A) methylpentanamine
B) dimethylpentanamine
C) 2,N-Dimethyl-3-pentanamine
D) N-methyl-2-methylpentanamine

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
What is the product if butanoic acid reacts with ammonia?
A) a secondary amine
B) the ammonium salt
C) a butane compound with a nitrogen attached to each carbon
D) a cyclic compound with one nitrogen and four carbons in the ring
A) a secondary amine
B) the ammonium salt
C) a butane compound with a nitrogen attached to each carbon
D) a cyclic compound with one nitrogen and four carbons in the ring

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
Diproylamine and HBr react to produce
A) dipropylammonium bromide.
B) dipropyl bromide.
C) propylammonium bromide.
D) ammonium bromide.
A) dipropylammonium bromide.
B) dipropyl bromide.
C) propylammonium bromide.
D) ammonium bromide.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
Amine salts formed from amines are mainly used in the drug industry because
A) they are acidic and react with bases.
B) they do not have a strong, unpleasant odor as do amines.
C) they are weak acids.
D) they are more soluble in water and blood fluids than the amines.
A) they are acidic and react with bases.
B) they do not have a strong, unpleasant odor as do amines.
C) they are weak acids.
D) they are more soluble in water and blood fluids than the amines.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
Salts of quaternary (4º) ammonium salts
A) have low boiling points.
B) have high melting points.
C) have weak covalent bonds.
D) are liquids at room temperature.
A) have low boiling points.
B) have high melting points.
C) have weak covalent bonds.
D) are liquids at room temperature.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
An amine will be converted into its conjugate acid by
A) application of heat.
B) reaction with an oxidizing agent.
C) reaction with a strong acid.
D) reaction with a strong base.
A) application of heat.
B) reaction with an oxidizing agent.
C) reaction with a strong acid.
D) reaction with a strong base.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
Predict the organic product formed when heat is applied to the compound below: 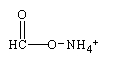
A)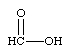
B)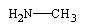
C)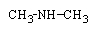
D)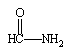
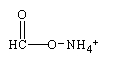
A)
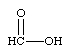
B)
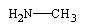
C)
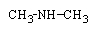
D)
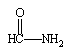

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
The IUPAC name of the molecule below is 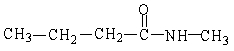
A) N-methylbutanamide
B) methylbutyramide
C) methylbutylamide
D) N-methyl-1-butylamine
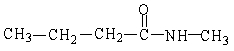
A) N-methylbutanamide
B) methylbutyramide
C) methylbutylamide
D) N-methyl-1-butylamine

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
The products of the hydrolysis reaction below are 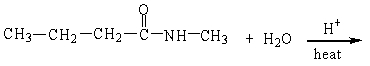
A)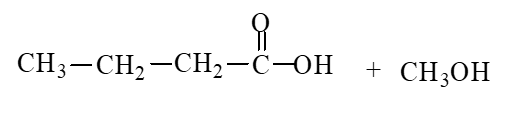
B)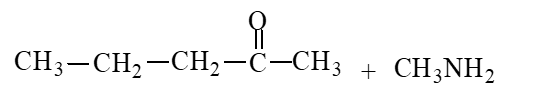
C)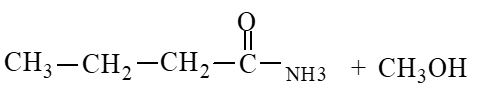
D)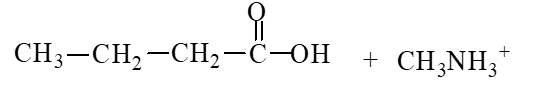
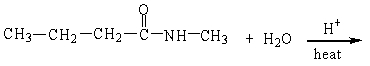
A)
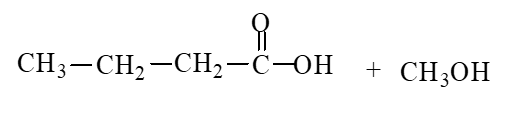
B)
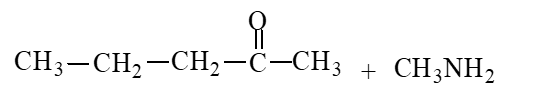
C)

D)
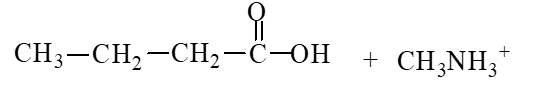

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
An amide can be identified by noting that
A) there is a nitrogen incorporated in the structure.
B) there is a nitrogen attached to an oxygen from a carbon.
C) all of the carbon chains attach to a nitrogen.
D) a nitrogen attaches to a carbonyl carbon.
A) there is a nitrogen incorporated in the structure.
B) there is a nitrogen attached to an oxygen from a carbon.
C) all of the carbon chains attach to a nitrogen.
D) a nitrogen attaches to a carbonyl carbon.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
The larger the carboxylic acid, the more hydrogen bonding occurs between molecules.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
The smell of rancid butter is due to the presence of butanoic acid.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
A common feature of carboxylic acids and phenols is that they contain an O-H group.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
When a carboxylic acid or phenol reacts with a strong base, one of the products is the conjugate base of the carboxylic acid or the phenol.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
Carboxylic acids have lower boiling points than esters of similar size.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
A 4º ammonium ion has four carbon atoms attached to a nitrogen atom causing it to have a 1+ charge.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
Water soluble amines form solutions with a pH that is greater than 7.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
Phenol forms a basic solution in water.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
Alcohols are not generally considered to be acids because they cannot donate a(n) ___ ion.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
Determine the major organic product of the following reaction:
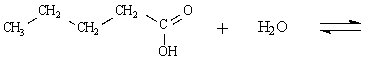
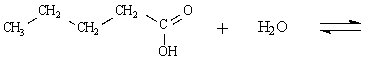

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
Determine the major organic product of the following reaction:
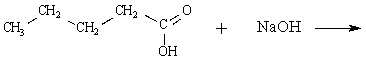


Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
Determine the major organic product of the following reaction:
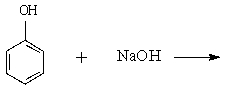
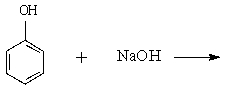

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
Generally, carboxylates are ___ in water if they contain fewer than 12 carbon atoms.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
A ___ amine is not capable of forming hydrogen bonds with a similar amine.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
A ____ amine is a ring compound in which at least one atom in the ring is nitrogen.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
Determine the major organic product of the following reaction:
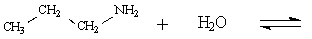
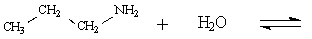

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
Determine the major organic product of the following reaction:
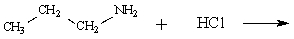
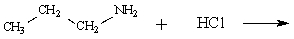

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


