Deck 7: Acids, Bases, and Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/91
Play
Full screen (f)
Deck 7: Acids, Bases, and Equilibrium
1
Which of the following is not a common characteristic of an acid?
A) it turns litmus pink/red
B) it tastes bitter
C) it dissolves common metals
D) all of these choices are common characteristics of acids
A) it turns litmus pink/red
B) it tastes bitter
C) it dissolves common metals
D) all of these choices are common characteristics of acids
it tastes bitter
2
Which of the following is not a common characteristic of a base?
A) it turns litmus pink/red
B) it feels slippery
C) it tastes bitter
D) all of these choices are common characteristics of bases
A) it turns litmus pink/red
B) it feels slippery
C) it tastes bitter
D) all of these choices are common characteristics of bases
it turns litmus pink/red
3
One characteristic of basic solution is that
A) the solution would turn litmus red.
B) the solution would have a slippery feel to it.
C) the solution would have a sour taste.
D) the solution would dissolve some metals.
A) the solution would turn litmus red.
B) the solution would have a slippery feel to it.
C) the solution would have a sour taste.
D) the solution would dissolve some metals.
the solution would have a slippery feel to it.
4
A conjugate acid-base pair
A) is related by the loss and gain of H+ between 2 corresponding compounds on opposite sides of the reaction arrow.
B) has the base donating an H+ to form its conjugate base.
C) has the acid donating an H+ to form its conjugate acid.
D) is not related in any way.
A) is related by the loss and gain of H+ between 2 corresponding compounds on opposite sides of the reaction arrow.
B) has the base donating an H+ to form its conjugate base.
C) has the acid donating an H+ to form its conjugate acid.
D) is not related in any way.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the conjugate acid of NH3?
A) NH2-
B) NH4+
C) HNO3
D) H3O+
A) NH2-
B) NH4+
C) HNO3
D) H3O+

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
6
In the reaction:
 The conjugate acid of water is ___.
The conjugate acid of water is ___.
A) HNO2
B) H2O
C) H3O+
D) NO2-
 The conjugate acid of water is ___.
The conjugate acid of water is ___.A) HNO2
B) H2O
C) H3O+
D) NO2-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
7
In the reaction:
 NO2- is the
NO2- is the
A) conjugate base of H2O.
B) conjugate base of HNO2.
C) conjugate acid of HNO2.
D) conjugate base of H3O+.
 NO2- is the
NO2- is theA) conjugate base of H2O.
B) conjugate base of HNO2.
C) conjugate acid of HNO2.
D) conjugate base of H3O+.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the conjugate base of the acid, carbonic acid? 
A) H2CO3
B) H2O
C) H3O+
D) HCO3-

A) H2CO3
B) H2O
C) H3O+
D) HCO3-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
9
Hydrogen sulfide ion, HS-, can react differently depending on the acidity of the solution in which it is present.
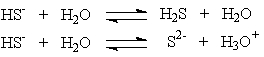 These two solutions show that the
These two solutions show that the
A) hydrogen sulfide ion is an acid.
B) hydrogen sulfide ion is a base.
C) hydrogen sulfide ion is amphoteric.
D) hydrogen sulfide ion can only react with water.
 These two solutions show that the
These two solutions show that theA) hydrogen sulfide ion is an acid.
B) hydrogen sulfide ion is a base.
C) hydrogen sulfide ion is amphoteric.
D) hydrogen sulfide ion can only react with water.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the conjugate acid of the bicarbonate ion, HCO3-?
A) H2CO3
B) CO32-
C) CO2
D) H3O+
A) H2CO3
B) CO32-
C) CO2
D) H3O+

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the conjugate base of the bicarbonate ion, HCO3-?
A) H2CO3
B) CO32-
C) CO2
D) H3O+
A) H2CO3
B) CO32-
C) CO2
D) H3O+

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
12
In the equation: 
A) H2CO3 and H3O+ are conjugate pairs
B) H2CO3 is not amphoteric
C) H2CO3 and HCO3 - are conjugate pairs
D) H2CO3 and H2O are conjugate pairs

A) H2CO3 and H3O+ are conjugate pairs
B) H2CO3 is not amphoteric
C) H2CO3 and HCO3 - are conjugate pairs
D) H2CO3 and H2O are conjugate pairs

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is the correct statement concerning the equation below? 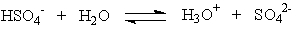
A) HSO4- is the conjugate base of SO4-
B) HSO4- is the conjugate base of H3O+
C) H2O is the conjugate base of HSO4-
D) SO42- is the conjugate base of HSO4-

A) HSO4- is the conjugate base of SO4-
B) HSO4- is the conjugate base of H3O+
C) H2O is the conjugate base of HSO4-
D) SO42- is the conjugate base of HSO4-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
14
Whenever an equilibrium constant, Keq, has a value greater than 1, which of the following statements is true at equilibrium?
A) The concentration of reactants is greater than the concentration of the products.
B) The concentration of products is the same as the concentration of reactants.
C) The concentration of the products is greater than the concentration of the reactants.
D) Relative composition of reaction mixture cannot be predicted.
A) The concentration of reactants is greater than the concentration of the products.
B) The concentration of products is the same as the concentration of reactants.
C) The concentration of the products is greater than the concentration of the reactants.
D) Relative composition of reaction mixture cannot be predicted.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
15
When a reaction is at equilibrium,
A) it has no products.
B) the rate of the forward reaction is equal to the rate of its reverse.
C) it occurs very quickly using up all of the reactants.
D) it produces the same amount of product as reactant.
A) it has no products.
B) the rate of the forward reaction is equal to the rate of its reverse.
C) it occurs very quickly using up all of the reactants.
D) it produces the same amount of product as reactant.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
16
Choose the equilibrium constant that indicates the greatest relative amount of reactant concentration at equilibrium.
A) 1.1 x 10-7
B) 2.3 x 107
C) 6.7 x 102
D) 8.3 x 10-2
A) 1.1 x 10-7
B) 2.3 x 107
C) 6.7 x 102
D) 8.3 x 10-2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
17
The equation:
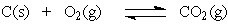 has the following equilibrium constant expression.
has the following equilibrium constant expression.
A)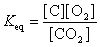
B)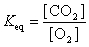
C)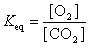
D)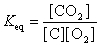
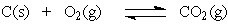 has the following equilibrium constant expression.
has the following equilibrium constant expression.A)
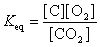
B)
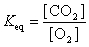
C)
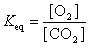
D)
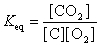

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
18
The equilibrium constant, Keq, for the ammonia synthesis below is found to be 6.0 x 10-2 at 500 C. Which of the following statements is true at equilibrium? 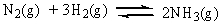
A) Product concentration is greater than reactant concentration.
B) Reactant concentration is greater than product concentration.
C) Reactant concentration is the same as product concentration.
D) Relative concentrations of reactants and products cannot be predicted.
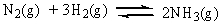
A) Product concentration is greater than reactant concentration.
B) Reactant concentration is greater than product concentration.
C) Reactant concentration is the same as product concentration.
D) Relative concentrations of reactants and products cannot be predicted.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
19
The equilibrium constant Keq for the reaction of molecular hydrogen and molecular iodine in the gas phase is 54.3 at 430 C.
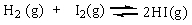 Which of the following statements is true for this reaction at equilibrium?
Which of the following statements is true for this reaction at equilibrium?
A) The reactant concentration is larger than the product concentration.
B) The product concentration is larger than the reactant concentration.
C) The reactant concentration is the same as the product concentration.
D) None of these answer choices are correct.
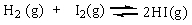 Which of the following statements is true for this reaction at equilibrium?
Which of the following statements is true for this reaction at equilibrium?A) The reactant concentration is larger than the product concentration.
B) The product concentration is larger than the reactant concentration.
C) The reactant concentration is the same as the product concentration.
D) None of these answer choices are correct.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
20
When stress is applied to a system at equilibrium such that the equilibrium is disturbed, the reaction proceeds in the direction that counteracts the disturbance. This explanation best describes ___.
A) Brønsted-Lowry concept
B) Ion-Ion attraction
C) Conjugate pair analysis
D) Le Châtelier's principle
A) Brønsted-Lowry concept
B) Ion-Ion attraction
C) Conjugate pair analysis
D) Le Châtelier's principle

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
21
The equation:
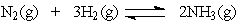 has the following equilibrium constant expression:
has the following equilibrium constant expression:
A)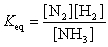
B)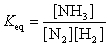
C)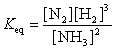
D)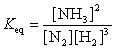
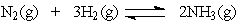 has the following equilibrium constant expression:
has the following equilibrium constant expression:A)
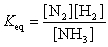
B)
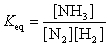
C)
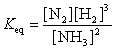
D)
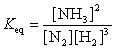

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
22
The effect of a catalyst on an equilibrium is
A) to speed up the reaction to the left.
B) to speed up the reaction to the right.
C) to speed up the reaction equally in both directions.
D) to shift the reaction in the direction of the catalyst.
A) to speed up the reaction to the left.
B) to speed up the reaction to the right.
C) to speed up the reaction equally in both directions.
D) to shift the reaction in the direction of the catalyst.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
23
In the Haber process for the production of ammonia,
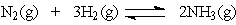 Which will not increase the amount of ammonia at equilibrium?
Which will not increase the amount of ammonia at equilibrium?
A) increasing the concentration of N2
B) increasing the concentration of H2
C) increasing the concentration of N2 and H2
D) addition of a catalyst
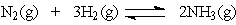 Which will not increase the amount of ammonia at equilibrium?
Which will not increase the amount of ammonia at equilibrium?A) increasing the concentration of N2
B) increasing the concentration of H2
C) increasing the concentration of N2 and H2
D) addition of a catalyst

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the conditions below would drive this reaction to the right?
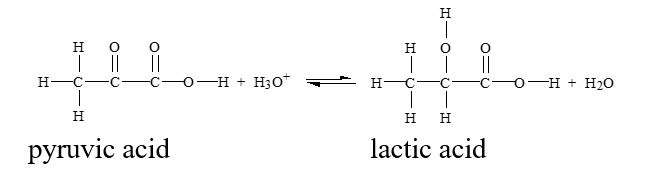
A) removing hydronium ion
B) adding lactic acid
C) adding water
D) adding pyruvic acid

A) removing hydronium ion
B) adding lactic acid
C) adding water
D) adding pyruvic acid

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements is true?
A) A catalyst used in a system that reaches a point of equilibrium decreases the reverse reactions and increases the forward reactions.
B) A catalyst used in a reversible reaction decreases the forward reactions but increases the reverse reactions.
C) A catalyst used in a reversible reaction increases the rate of both the forward and reverse reactions to the same extent but does not change the point of equilibrium.
D) A catalyst used in a system that reaches a point of equilibrium changes the point of equilibrium and decreases the rate of forward reactions only.
A) A catalyst used in a system that reaches a point of equilibrium decreases the reverse reactions and increases the forward reactions.
B) A catalyst used in a reversible reaction decreases the forward reactions but increases the reverse reactions.
C) A catalyst used in a reversible reaction increases the rate of both the forward and reverse reactions to the same extent but does not change the point of equilibrium.
D) A catalyst used in a system that reaches a point of equilibrium changes the point of equilibrium and decreases the rate of forward reactions only.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
26
In the reaction below:
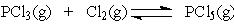 Increasing the concentration of Cl2 will ___ at equilibrium.
Increasing the concentration of Cl2 will ___ at equilibrium.
A) increase the concentration of PCl5
B) increase the concentration of PCl3
C) decrease the concentration of PCl5
D) have no effect
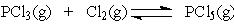 Increasing the concentration of Cl2 will ___ at equilibrium.
Increasing the concentration of Cl2 will ___ at equilibrium.A) increase the concentration of PCl5
B) increase the concentration of PCl3
C) decrease the concentration of PCl5
D) have no effect

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
27
What is the concentration of [H3O+] in an aqueous solution when the [OH-] is 5.2 x 10-9 M?
A) 1.9 x 10-6 M
B) 5.7 M
C) 1.0 x 10-14 M
D) 9.8 x 10-9 M
A) 1.9 x 10-6 M
B) 5.7 M
C) 1.0 x 10-14 M
D) 9.8 x 10-9 M

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the [OH-] in an aqueous solution when the [H3O+] is 1.2 x 10-2M.
A) 3.8 x 10-10 M
B) 8.3 x 10-11 M
C) 2.0 x 10-10 M
D) 1.0 x 10-14 M
A) 3.8 x 10-10 M
B) 8.3 x 10-11 M
C) 2.0 x 10-10 M
D) 1.0 x 10-14 M

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
29
Kw, the equilibrium constant for the ionization of water by the equation below, is 1.0 x 10-14. What does that mean when we are considering pure water? 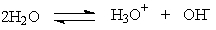
A) More ions exist than water molecules.
B) The majority of the molecules present are in the form of H2O.
C) The amount of water is the same as the amount of the ions present.
D) There will always be more hydronium ions present than water at equilibrium.
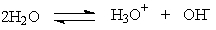
A) More ions exist than water molecules.
B) The majority of the molecules present are in the form of H2O.
C) The amount of water is the same as the amount of the ions present.
D) There will always be more hydronium ions present than water at equilibrium.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
30
A solution in which the concentration of H+ is greater than the concentration of OH- will
A) have a pH greater than 7.0 and be basic.
B) have a pH less than 7.0 and be basic.
C) have a pH greater than 7.0 and be acidic.
D) have a pH less than 7.0 and be acidic.
A) have a pH greater than 7.0 and be basic.
B) have a pH less than 7.0 and be basic.
C) have a pH greater than 7.0 and be acidic.
D) have a pH less than 7.0 and be acidic.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
31
Basic solutions have an H+ concentration
A) greater than the OH- concentration and a pH greater than 7.0.
B) greater than the OH- concentration and a pH less than 7.0.
C) less than the OH- concentration and a pH greater than 7.0.
D) less than the OH- concentration and a pH less than 7.0.
A) greater than the OH- concentration and a pH greater than 7.0.
B) greater than the OH- concentration and a pH less than 7.0.
C) less than the OH- concentration and a pH greater than 7.0.
D) less than the OH- concentration and a pH less than 7.0.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
32
Acidic solutions have an H+ concentration
A) greater than the OH- concentration and a pH greater than 7.0.
B) greater than the OH- concentration and a pH less than 7.0.
C) less than the OH- concentration and a pH greater than 7.0.
D) less than the OH- concentration and a pH less than 7.0.
A) greater than the OH- concentration and a pH greater than 7.0.
B) greater than the OH- concentration and a pH less than 7.0.
C) less than the OH- concentration and a pH greater than 7.0.
D) less than the OH- concentration and a pH less than 7.0.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
33
One liter of a solution is found to contain 0.022 moles of HCl. Calculate the pH of the solution. Assume complete dissociation.
A) pH = 0.022
B) pH = 1.00
C) pH = 2.2
D) pH = 1.66
A) pH = 0.022
B) pH = 1.00
C) pH = 2.2
D) pH = 1.66

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
34
When one is referring to pH, what is the meaning of the p?
A) Take the log of the number.
B) Multiply the p constant by the number.
C) Take the negative log of the number.
D) All are possible depending on the number following the p.
A) Take the log of the number.
B) Multiply the p constant by the number.
C) Take the negative log of the number.
D) All are possible depending on the number following the p.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
35
What is the pH of a solution in which [H3O]+ is 2.2 x 10-12 M?
A) 2.34
B) 4.54 x 10-3
C) 11.66
D) 8.42
A) 2.34
B) 4.54 x 10-3
C) 11.66
D) 8.42

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
36
When we talk about pH we are referring to the concentration of hydronium ions present. If the concentration of hydronium in a solution is 1.0 x 10-8 M, what is the hydroxide ion concentration?
A) 1.0 x 10-6 M
B) 9.0 x 10-8 M
C) 1.0 x 10-14 M
D) The concentration cannot be predicted.
A) 1.0 x 10-6 M
B) 9.0 x 10-8 M
C) 1.0 x 10-14 M
D) The concentration cannot be predicted.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the pH of solution produced by dissolving 0.001 moles of HNO3 in a liter of water. Assume complete dissociation.
A) pH = 3.0
B) pH = 0.001
C) pH = 1 x 10-3
D) pH = 1 x 103
A) pH = 3.0
B) pH = 0.001
C) pH = 1 x 10-3
D) pH = 1 x 103

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
38
What is the molar concentration of hydronium ions in a sample of a soft drink that has a pH of 4?
A) 1/4 M
B) 4 M
C) 1 x 104 M
D) 1 x 10-4 M
A) 1/4 M
B) 4 M
C) 1 x 104 M
D) 1 x 10-4 M

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
39
You produce 500 mL of a 0.001 M HClO4, which ionizes completely in water. What is the pH you should expect?
A) pH = 0.5
B) pH = 3.0
C) pH = 2.7
D) pH = 500
A) pH = 0.5
B) pH = 3.0
C) pH = 2.7
D) pH = 500

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
40
A sample of orange juice has a pH of 3.44. Its [H3O+] is ___.
A) 5.4 x 10-1 M
B) 3.6 x 10-4 M
C) 1.8 x 10-5
D) 2.7 x 10-3
A) 5.4 x 10-1 M
B) 3.6 x 10-4 M
C) 1.8 x 10-5
D) 2.7 x 10-3

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
41
What is the [H3O+] of an antacid tablet solution with a pH of 8.32?
A) 4.8 x 10-9 M
B) 5.7 x 10-9 M
C) 2.4 x 10-7 M
D) 1.2 x 10-6 M
A) 4.8 x 10-9 M
B) 5.7 x 10-9 M
C) 2.4 x 10-7 M
D) 1.2 x 10-6 M

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the pH of a solution containing 0.15 moles KOH dissolved in enough water to produce 2 liters of solution.
A) pH = 0.075
B) pH = 0.15
C) pH = 1.12
D) pH = 12.88
A) pH = 0.075
B) pH = 0.15
C) pH = 1.12
D) pH = 12.88

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
43
A Ka can be calculated for some chemical reactions. The Ka is
A) the Keq for the reaction to the right.
B) the Keq for the reaction to the left.
C) the Keq for the dissociation of an acid.
D) the pH of a very weak solution.
A) the Keq for the reaction to the right.
B) the Keq for the reaction to the left.
C) the Keq for the dissociation of an acid.
D) the pH of a very weak solution.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements is true?
A) The larger the Ka for an acid, the weaker the acid.
B) The smaller the pKa of an acid, the stronger the acid.
C) The larger the pKa of an acid, the stronger the acid.
D) The smaller the Ka, the stronger the acid.
A) The larger the Ka for an acid, the weaker the acid.
B) The smaller the pKa of an acid, the stronger the acid.
C) The larger the pKa of an acid, the stronger the acid.
D) The smaller the Ka, the stronger the acid.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
45
What is the [OH-] of a solution whose pH is 3.00?
A) 1.0 x 10-3 M
B) 1.0 x 10-14 M
C) 1.0 x 10-11 M
D) 1.0 x 10-12 M
A) 1.0 x 10-3 M
B) 1.0 x 10-14 M
C) 1.0 x 10-11 M
D) 1.0 x 10-12 M

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
46
Which material would be effective for neutralizing a minor acid spill?
A) soap solution
B) vinegar solution
C) baking soda
D) household ammonia
A) soap solution
B) vinegar solution
C) baking soda
D) household ammonia

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
47
15.00 mL of 0.100 M NaOH is required to completely neutralize 25.00 mL of an HCl solution. What is the concentration of the HCl solution?
A) 0.0600 M
B) 0.100 M
C) 0.167 M
D) 1.50 M
A) 0.0600 M
B) 0.100 M
C) 0.167 M
D) 1.50 M

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
48
What volume of 0.200 M HCl is required to completely neutralize 50.00 mL of 0.150 M KOH?
A) 7.50 mL
B) 50.0 mL
C) 66.7 mL
D) 37.5 mL
A) 7.50 mL
B) 50.0 mL
C) 66.7 mL
D) 37.5 mL

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
49
The pH of blood is held reasonably stable by which buffer system?
A) H3PO4/H2PO4-
B) NaCl/Cl-
C) H2CO3/HCO3-
D) HCl/Cl-
A) H3PO4/H2PO4-
B) NaCl/Cl-
C) H2CO3/HCO3-
D) HCl/Cl-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
50
Excessive use of antacids can lead to
A) an increase in blood pH known as acidosis.
B) an increase in blood pH known as alkalosis.
C) a decrease in blood pH know as acidosis.
D) a decrease in blood pH known as alkylosis.
A) an increase in blood pH known as acidosis.
B) an increase in blood pH known as alkalosis.
C) a decrease in blood pH know as acidosis.
D) a decrease in blood pH known as alkylosis.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
51
The ethylammonium ion, CH3CH2NH3+ has a pKa of 10.81. It reacts with water to form ethylamine, CH3CH2NH2 and H3O+ as shown below.
 Which of the following statements is true at pH 7?
Which of the following statements is true at pH 7?
A) ethylammonium ion predominates
B) ethylamine predominates
C) the concentration of ethylamine equals that of ethylammonium ion
D) the pH is higher than pKa of the ethylammonium ion
 Which of the following statements is true at pH 7?
Which of the following statements is true at pH 7?A) ethylammonium ion predominates
B) ethylamine predominates
C) the concentration of ethylamine equals that of ethylammonium ion
D) the pH is higher than pKa of the ethylammonium ion

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
52
The carbonic acid/bicarbonate buffer is important in maintaining the proper pH of human blood.
 Diarrhea can lead to the loss of HCO3-. According to LeChatelier's principle, removal of HCO3- will shift the above equilibrium to the ___ and result in a(n) ___ in blood pH.
Diarrhea can lead to the loss of HCO3-. According to LeChatelier's principle, removal of HCO3- will shift the above equilibrium to the ___ and result in a(n) ___ in blood pH.
A) left/decrease
B) right/decrease
C) left/increase
D) right/increase
 Diarrhea can lead to the loss of HCO3-. According to LeChatelier's principle, removal of HCO3- will shift the above equilibrium to the ___ and result in a(n) ___ in blood pH.
Diarrhea can lead to the loss of HCO3-. According to LeChatelier's principle, removal of HCO3- will shift the above equilibrium to the ___ and result in a(n) ___ in blood pH.A) left/decrease
B) right/decrease
C) left/increase
D) right/increase

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
53
The Ka for the reaction of acetic acid and water shown below is 1.8 x 10-5.
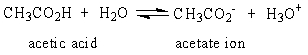 Which of the following statements is true at pH 7?
Which of the following statements is true at pH 7?
A) there is much more acetic acid than acetate ion
B) there is more acetate ion than acetic acid
C) the concentration of acetate ion is equal to that of acetic acid
D) the pH is lower than pKa of acetic acid
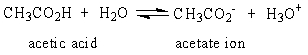 Which of the following statements is true at pH 7?
Which of the following statements is true at pH 7?A) there is much more acetic acid than acetate ion
B) there is more acetate ion than acetic acid
C) the concentration of acetate ion is equal to that of acetic acid
D) the pH is lower than pKa of acetic acid

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
54
The pH of blood is maintained at 7.35-7.45 by the following buffer system:
 The H2CO3 is produced by the reaction of CO2 with water according to the equation:
The H2CO3 is produced by the reaction of CO2 with water according to the equation:
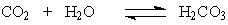 When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?
When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?
A) The pH will increase.
B) The pH will decrease.
C) The pH will remain the same.
D) It is impossible to predict the effect.
 The H2CO3 is produced by the reaction of CO2 with water according to the equation:
The H2CO3 is produced by the reaction of CO2 with water according to the equation: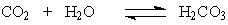 When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?
When one exercises, there is increased cellular output of CO2. What effect will this have on the pH of the blood if the excess CO2 is not eliminated?A) The pH will increase.
B) The pH will decrease.
C) The pH will remain the same.
D) It is impossible to predict the effect.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
55
Antacids may contain which ion to reduce acidity?
A) Na+
B) CO32-
C) Al3+
D) Cl-
A) Na+
B) CO32-
C) Al3+
D) Cl-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
56
Acids and bases can react with and damage many compounds that are vital to living organisms.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
57
HNO3 can act as acid and as a base.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
58
The higher the numerical value of an equilibrium constant (Keq), the further to the right the reaction will proceed.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
59
The greater the hydronium ion concentration, the higher the pH.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
60
For a buffer to continue to work effectively, the pH of a buffer has to be close to the pKa of the conjugate acid.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
61
A buffer is capable of reducing the effect of the addition of small amounts of hydronium or hydroxide ion to a solution.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
62
If a wine has fermented beyond the step that produces alcohol, it becomes acidic, making the wine taste ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
63
Basic substances tend to have a ___ taste.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
64
The formula of the conjugate base of HClO4 is ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
65
A base is a compound that produces an ion with the formula ___ in aqueous solution and an acid is a compound that produces the ___ ion in aqueous solution, which is also written as the hydronium ion (___).

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
66
Compounds that can act as acids and bases are called ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
67
The conjugate base of the hydrogen sulfate ion (HSO4-) is ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
68
The conjugate acid of the hydrogen sulfate ion (HSO4-) is ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
69
In the following equation, identify the acid, base, conjugate acid, and conjugate base.



Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
70
Increasing the concentration of a ___ at equilibrium will drive the system toward the reactants.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
71
A is red, B is colorless, and C is yellow. Initially the system is in equilibrium and has an orange color.
 What color will the system become if B is removed?
What color will the system become if B is removed?
 What color will the system become if B is removed?
What color will the system become if B is removed?
Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
72
A is red, B is colorless, and C is yellow. Initially the system is in equilibrium and has an orange color.
 What color will the system become if B is added?
What color will the system become if B is added?
 What color will the system become if B is added?
What color will the system become if B is added?
Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
73
A is red, B is colorless, and C is yellow. Initially the system is in equilibrium and has an orange color.
 What color will the system become if it is heated?
What color will the system become if it is heated?
 What color will the system become if it is heated?
What color will the system become if it is heated?
Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
74
A is red, B is colorless, and C is yellow. Initially the system is in equilibrium and has an orange color.
 What color will the system become if concentration of A is increased?
What color will the system become if concentration of A is increased?
 What color will the system become if concentration of A is increased?
What color will the system become if concentration of A is increased?
Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
75
A sample of blood has a pH of 7.37, which means that the sample of blood is slightly ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
76
Nitrous acid (HNO2) has Ka = 4.0 x 10-4, and carbonic acid (H2CO3) has Ka= 4.4 x 10-7. The acid with a stronger conjugate base is ___

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
77
The symptoms of alkalosis appear when blood serum pH is ___.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
78
____ is a condition in which the pH of blood is below the normal range.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following are the strong acids? List all.
HF, HNO3, HCN, CH3COOH, H2SO4
HF, HNO3, HCN, CH3COOH, H2SO4

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
80
Calculate the pH of a 0.0019 M HNO3 solution.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck


