Exam 7: Acids, Bases, and Equilibrium
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
The carbonic acid/bicarbonate buffer is important in maintaining the proper pH of human blood.
 Diarrhea can lead to the loss of HCO3-. According to LeChatelier's principle, removal of HCO3- will shift the above equilibrium to the ___ and result in a(n) ___ in blood pH.
Diarrhea can lead to the loss of HCO3-. According to LeChatelier's principle, removal of HCO3- will shift the above equilibrium to the ___ and result in a(n) ___ in blood pH.
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
B
One characteristic of basic solution is that
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
B
One liter of a solution is found to contain 0.022 moles of HCl. Calculate the pH of the solution. Assume complete dissociation.
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
D
The equilibrium constant Keq for the reaction of molecular hydrogen and molecular iodine in the gas phase is 54.3 at 430 C.
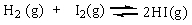 Which of the following statements is true for this reaction at equilibrium?
Which of the following statements is true for this reaction at equilibrium?
(Multiple Choice)
4.9/5  (35)
(35)
Which material would be effective for neutralizing a minor acid spill?
(Multiple Choice)
4.9/5  (42)
(42)
Choose the equilibrium constant that indicates the greatest relative amount of reactant concentration at equilibrium.
(Multiple Choice)
4.7/5  (33)
(33)
In the Haber process for the production of ammonia,
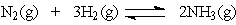 Which will not increase the amount of ammonia at equilibrium?
Which will not increase the amount of ammonia at equilibrium?
(Multiple Choice)
4.9/5  (27)
(27)
The pH of blood is held reasonably stable by which buffer system?
(Multiple Choice)
4.7/5  (32)
(32)
A buffer is capable of reducing the effect of the addition of small amounts of hydronium or hydroxide ion to a solution.
(True/False)
4.8/5  (36)
(36)
Nitrous acid (HNO2) has Ka = 4.0 x 10-4, and carbonic acid (H2CO3) has Ka= 4.4 x 10-7. The acid with a stronger conjugate base is ___
(Short Answer)
4.7/5  (31)
(31)
You produce 500 mL of a 0.001 M HClO4, which ionizes completely in water. What is the pH you should expect?
(Multiple Choice)
4.8/5  (24)
(24)
Carrot juice has a pH of 5.1 while asparagus juice has a pH of 5.6. Are they both acidic or basic? Which is more so?
(Short Answer)
4.8/5  (42)
(42)
Which of the conditions below would drive this reaction to the right?
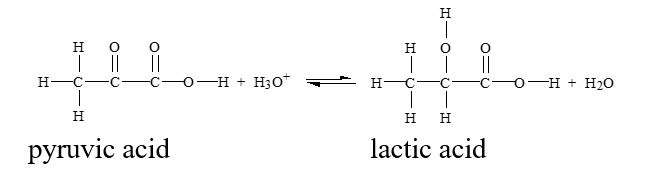
(Multiple Choice)
4.7/5  (27)
(27)
Showing 1 - 20 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)