Deck 4: An Introduction to Organic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 4: An Introduction to Organic Compounds
1
Which of the following statements is (are) true about hydrogen bonding interaction?
A) It occurs only in molecules that contain H bonded to a highly electronegative atom, such as N, O, or F.
B) It is a special type of dipole-dipole interaction.
C) It is present in H2O.
D) All of these choices.
A) It occurs only in molecules that contain H bonded to a highly electronegative atom, such as N, O, or F.
B) It is a special type of dipole-dipole interaction.
C) It is present in H2O.
D) All of these choices.
All of these choices.
2
The correct condensed structure of Propylene glycol, a compound used as a solvent for drugs is 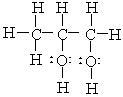
A) CH3CH2CH2OH
B) CH2HOHCOH2COH
C)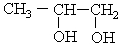
D)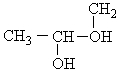

A) CH3CH2CH2OH
B) CH2HOHCOH2COH
C)
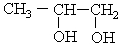
D)

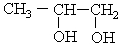
3
An isotope of fluoromethane, CH3F, has been used for cerebral blood flow measurement. Which of the following represent the correct line-bond structure of fluoromethane? (C is the central atom.)
A)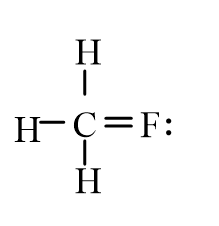
B)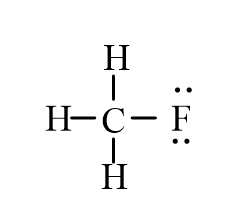
C)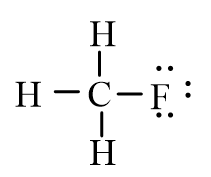
D)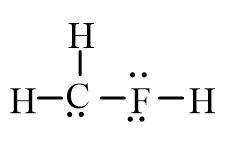
A)

B)

C)

D)
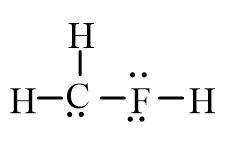

4
Dichloromethane, CH2Cl2, is widely used as a solvent. Which of the following represents its correct line-bond structure? In this molecule, carbon is the central atom.
A)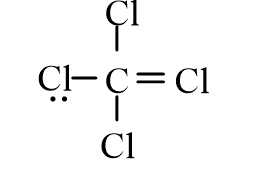
B)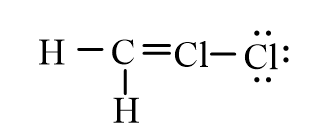
C)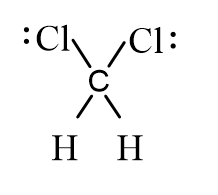
D)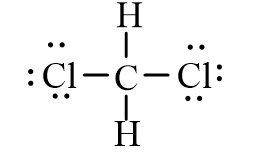
A)
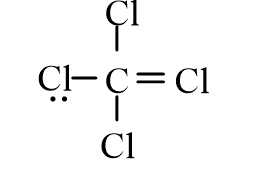
B)
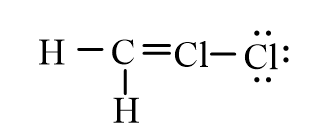
C)

D)
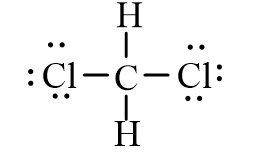

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following represents the correct line-bond structure of nitrosyl chloride (ONCl)? In this molecule, nitrogen is the central atom.
A)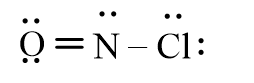
B)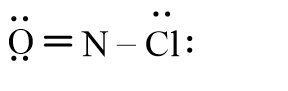
C)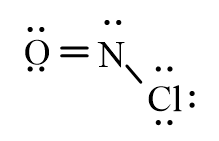
D)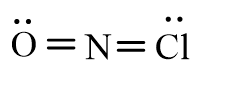
A)
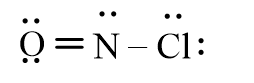
B)
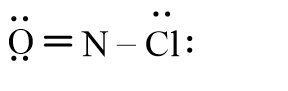
C)
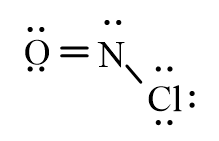
D)
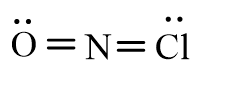

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the correct line-bond structure for the BrO3- ion?
A)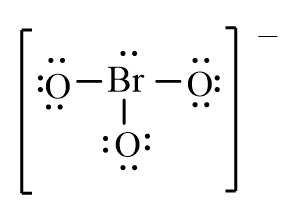
B)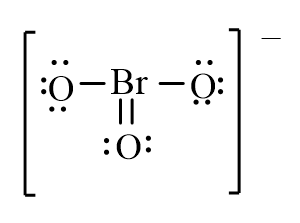
C)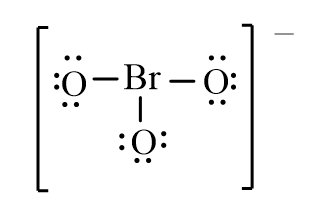
D)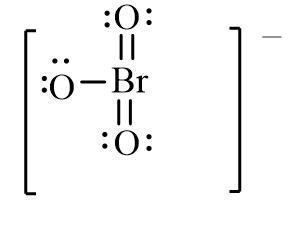
A)
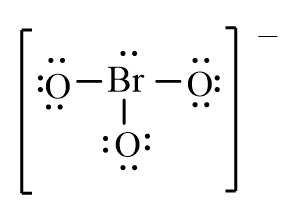
B)
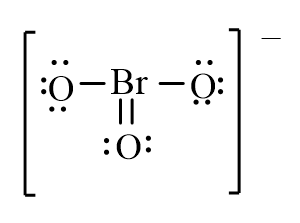
C)
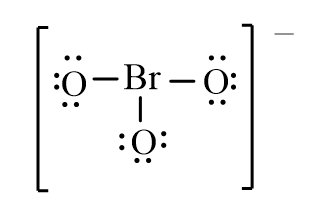
D)
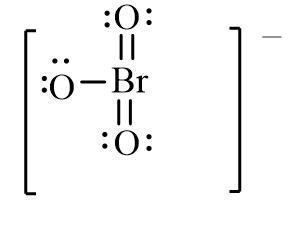

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
The correct line-bond structure for SO42- ion is
A)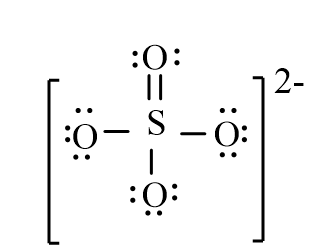
B)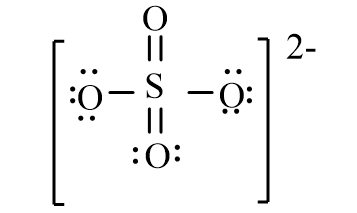
C)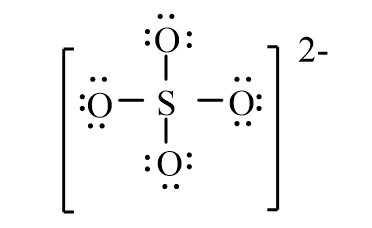
D)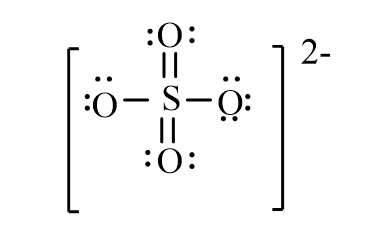
A)

B)
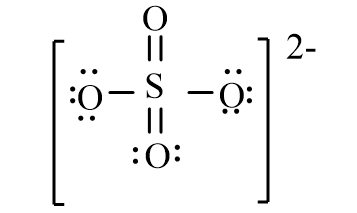
C)
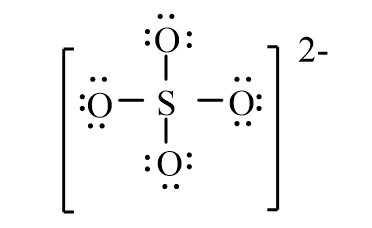
D)
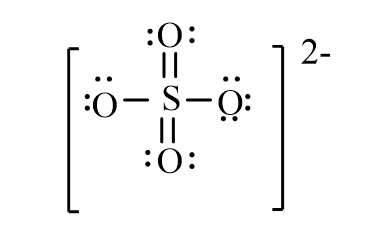

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
The correct line-bond structure for ClI2+ ion is
A)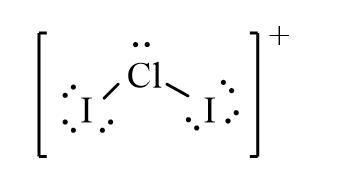
B)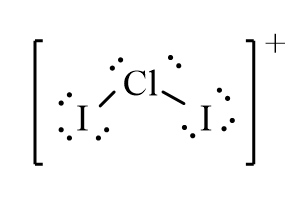
C)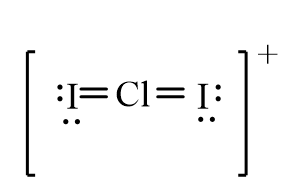
D)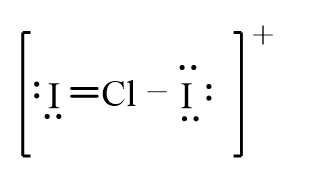
A)
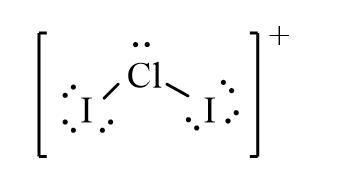
B)

C)
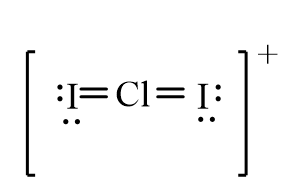
D)
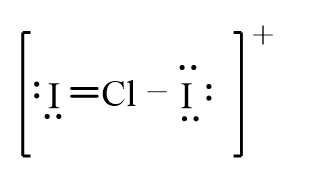

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following contains a nonpolar covalent bond?
A) LiBr
B) CaCl2
C) H2O
D) O2
A) LiBr
B) CaCl2
C) H2O
D) O2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following molecules is polar?
A) H2CO (hint: C is the central atom)
B) CH4
C) N2
D) BCl3 (hint: B is the central atom and doesn't have an octet)
A) H2CO (hint: C is the central atom)
B) CH4
C) N2
D) BCl3 (hint: B is the central atom and doesn't have an octet)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
The molecule CH4 has a tetrahedral shape. The angle between the bonds in CH4 is approximately ___.
A) 104.5
B) 109.5
C) 90
D) 120
A) 104.5
B) 109.5
C) 90
D) 120

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
The shape of formaldehyde CH2O (C is the central atom), a substance often used in building insulation materials, is ___.
A) trigonal planar
B) tetrahedral
C) linear
D) pyramidal
A) trigonal planar
B) tetrahedral
C) linear
D) pyramidal

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
Which molecule is nonpolar even though it contains polar bonds?
A) NH3
B) H2O
C) CCF4
D) CHClF2
A) NH3
B) H2O
C) CCF4
D) CHClF2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following bonds can be classified as polar?
A) NaCl
B) CH
C) CsBr
D) ClF
A) NaCl
B) CH
C) CsBr
D) ClF

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
Which bond is most polar?
A) B-Cl
B) C-Cl
C) O-Cl
D) Cl-Br
A) B-Cl
B) C-Cl
C) O-Cl
D) Cl-Br

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
Oxygen is more electronegative than carbon, so when an oxygen atom pulls the shared pair of bonding electrons toward itself to form a covalent bond, the oxygen atom carries
A) a partial positive charge.
B) no charge.
C) a partial negative charge.
D) both a partial positive charge and a negative charge.
A) a partial positive charge.
B) no charge.
C) a partial negative charge.
D) both a partial positive charge and a negative charge.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
In which bond does the oxygen atom possess a partial positive charge?
A) O-H
B) O-F
C) N-O
D) O-C
A) O-H
B) O-F
C) N-O
D) O-C

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
Which atoms in the following molecule would have - charge based on the electronegativities of the elements? 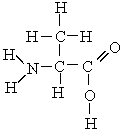
A) H and O
B) O and C
C) C and N
D) N and O

A) H and O
B) O and C
C) C and N
D) N and O

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
Which atoms in the following molecule would have - charge based on the electronegativities of the elements? 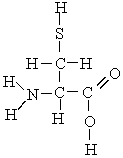
A) H, C, and O
B) N, O, and S
C) C and N
D) N, S, and C

A) H, C, and O
B) N, O, and S
C) C and N
D) N, S, and C

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
Which bond is least polar?
A) H-F
B) H-Cl
C) H-Br
D) H-I
A) H-F
B) H-Cl
C) H-Br
D) H-I

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
Which molecule has a linear geometry?
A) SO2
B) H2O
C) OF2
D) CO2
A) SO2
B) H2O
C) OF2
D) CO2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following molecules is nonpolar?
A)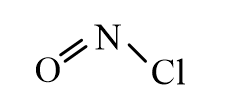
B)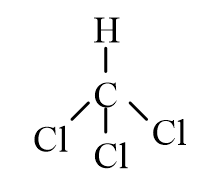
C)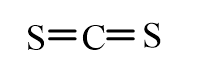
D)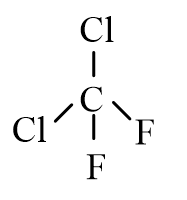
A)
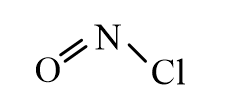
B)

C)
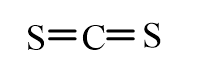
D)


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following molecules is polar?
A)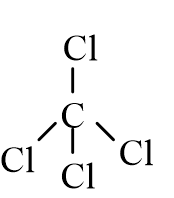
B)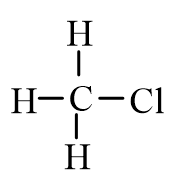
C)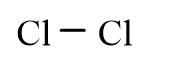
D)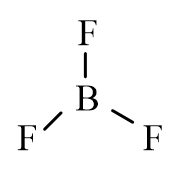
A)

B)

C)
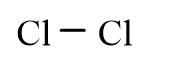
D)


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
What is the shape around C atom in OCN-?
A) linear
B) bent
C) pyramidal
D) Trigonal planar
A) linear
B) bent
C) pyramidal
D) Trigonal planar

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
The strongest noncovalent interaction that can occur between hydrocarbons is ___.
A) dipole-dipole forces
B) hydrogen bonding
C) ion-dipole interaction
D) London force
A) dipole-dipole forces
B) hydrogen bonding
C) ion-dipole interaction
D) London force

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
The strongest noncovalent interaction that can occur between two methyl alcohol molecules is ___. 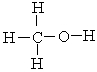
A) dipole-dipole forces
B) hydrogen bonding
C) ion-dipole interaction
D) London force

A) dipole-dipole forces
B) hydrogen bonding
C) ion-dipole interaction
D) London force

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
Organic compound must contain which of the following atoms?
A) O
B) H
C) S
D) C
A) O
B) H
C) S
D) C

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
The following molecule belongs to which family of compounds? 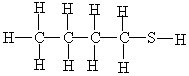
A) alcohols
B) Ethers
C) sulfides
D) Thiols

A) alcohols
B) Ethers
C) sulfides
D) Thiols

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
Which pairs of molecules can form a hydrogen bond with one another?
A) NF3
B) CH3CH2CH2CH2CH3
C) CH3CH2NH2
D) CCl4
A) NF3
B) CH3CH2CH2CH2CH3
C) CH3CH2NH2
D) CCl4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
Serotonin is a molecule involved in the transmission of nerve impulses. One of the organic families present in serotonin is ___. 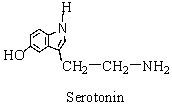
A) ester
B) alkyne
C) phenol
D) aldehyde

A) ester
B) alkyne
C) phenol
D) aldehyde

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
The following molecule belongs to which organic family? 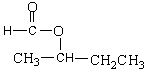
A) ketone
B) ether
C) carboxylic acid
D) ester
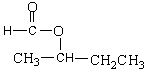
A) ketone
B) ether
C) carboxylic acid
D) ester

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
The CaF bond in CaF2 is nonpolar.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
Potassium has lower electronegativity than F.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
CH2Cl2 is polar.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
The Si-Si bond in Cl3SiSiCl3 is expected to be polar.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
Dioxygen difluoride whose actual three dimensional shape is provided below is non-polar.
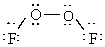
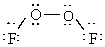

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
The kinds of interactions that exist between CH3COOH molecules include dipole-dipole (including hydrogen bonding) interactions and London forces.
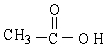
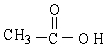

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
Hydrocarbons can take part in dipole-dipole interactions.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
The molecule shown below contains an aldehyde functional group.
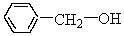
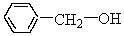

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
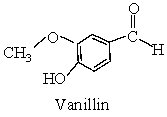
Ketone is present in Vanillin.


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
The shape of H2O is bent.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
Of the two elements, lithium and chromium, ___ has greater electronegativity.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
The ___ forces are the attraction of neighboring polar groups for one another.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
The organic family present in this molecule is a (an) ___.
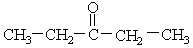
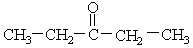

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
Which share the stronger London force interactions, two CH3CH2CH2CH2CH2CH3 molecules or two 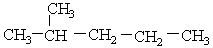 ?
?
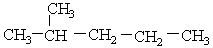 ?
?
Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
Sketch the line-bond structure of the NO3- ion. Specify its molecular geometry.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
Draw two disulfides with the formula C4H10S2.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
Pseudoephedrine is a nasal decongestant found in several cold remedies. Which organic families are present in this molecule?
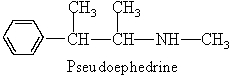
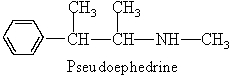

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
This molecule is responsible for the aroma of grape fruit. Which organic families are present?
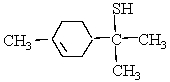


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
Draw two ethers that have the formula C4H10O.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
Draw two esters with the formula C5H10O

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
Draw a molecule with the formula C7H8O that has both aldehyde and aromatic groups.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
Choose the best matching for:
-CO2
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear
-CO2
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
Choose the best matching for:
-SO2
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear
-SO2
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
Choose the best matching for:
-CH4
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear
-CH4
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
Choose the best matching for:
-CH2O (Carbon is the central atom)
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear
-CH2O (Carbon is the central atom)
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
Choose the best matching for:
-NH3
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear
-NH3
A) Bent
B) pyramidal
C) Trigonal planar
D) Tetrahedral
E) linear

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
Choose the best matching for:
-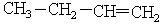
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-
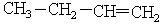
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
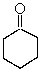
Choose the best matching for:
-
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-

A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
Choose the best matching for:
-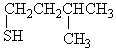
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-
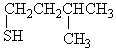
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
Choose the best matching for:
-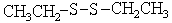
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-
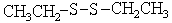
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
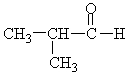
Choose the best matching for:
-
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-

A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
Choose the best matching for:
-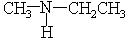
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-
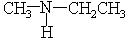
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
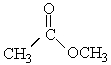
Choose the best matching for:
-
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-

A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
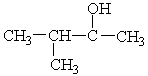
Choose the best matching for:
-
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-

A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
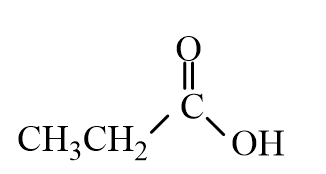
Choose the best matching for:
-
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-

A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
Choose the best matching for:
-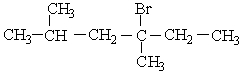
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-
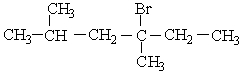
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
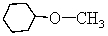
68
Choose the best matching for:
-
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-

A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
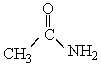
69
Choose the best matching for:
-
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-

A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
Choose the best matching for:
-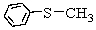
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol
-
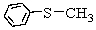
A) disulfide
B) thiol
C) aldehyde
D) sulfide
E) amine
F) ether
G) alkene
H) Amide
I) Carboxylic acid
J) ketone
K) ester
L) Alkyl halide
M) alcohol

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
Sketch the line-bond structure for each molecule or polyatomic ion of the following. Determine the molecular geometry and molecular polarity. (Indicate its shape and whether it is polar or nonpolar).
-H2S
A) Lewis Structure
B) Shape
C) Polar or nonpolar?
-H2S
A) Lewis Structure
B) Shape
C) Polar or nonpolar?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
Sketch the line-bond structure for each molecule or polyatomic ion of the following. Determine the molecular geometry and molecular polarity. (Indicate its shape and whether it is polar or nonpolar).
-AsCl3
A) Lewis Structure
B) Shape
C) Polar or nonpolar?
-AsCl3
A) Lewis Structure
B) Shape
C) Polar or nonpolar?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
Sketch the line-bond structure for each molecule or polyatomic ion of the following. Determine the molecular geometry and molecular polarity. (Indicate its shape and whether it is polar or nonpolar).
-CSe2
A) Lewis Structure
B) Shape
C) Polar or nonpolar?
-CSe2
A) Lewis Structure
B) Shape
C) Polar or nonpolar?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
Sketch the line-bond structure for each molecule or polyatomic ion of the following. Determine the molecular geometry and molecular polarity. (Indicate its shape and whether it is polar or nonpolar).
-PO43-
A) Lewis Structure
B) Shape
C) Polar or nonpolar?
-PO43-
A) Lewis Structure
B) Shape
C) Polar or nonpolar?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
Sketch the line-bond structure for each molecule or polyatomic ion of the following. Determine the molecular geometry and molecular polarity. (Indicate its shape and whether it is polar or nonpolar).
-NO2-
A) Lewis Structure
B) Shape
C) Polar or nonpolar?
-NO2-
A) Lewis Structure
B) Shape
C) Polar or nonpolar?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck


