Exam 4: An Introduction to Organic Compounds
Exam 1: Science and Measurements81 Questions
Exam 2: Atoms and Elements79 Questions
Exam 3: Compounds82 Questions
Exam 4: An Introduction to Organic Compounds75 Questions
Exam 5: Reactions90 Questions
Exam 6: Gases, Solutions, Colloids, and Suspensions104 Questions
Exam 7: Acids, Bases, and Equilibrium91 Questions
Exam 8: Organic Reactions Hydrocarbons, Carboxlic Acids, Amines, and Related Compounds77 Questions
Exam 9: Organic Reactions 2-Alcohols, Ethers, Aldehydes, and Ketones85 Questions
Exam 10: Carbohydrates84 Questions
Exam 11: Lipids and Membranes90 Questions
Exam 12: Peptides, Proteins, and Enzymes86 Questions
Exam 13: Nucleic Acids99 Questions
Exam 14: Metabolism84 Questions
Select questions type
Which of the following molecules is polar?
Free
(Multiple Choice)
4.7/5  (44)
(44)
Correct Answer:
B
Potassium has lower electronegativity than F.
Free
(True/False)
4.9/5  (33)
(33)
Correct Answer:
True
The correct condensed structure of Propylene glycol, a compound used as a solvent for drugs is 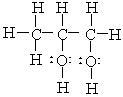
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following statements is (are) true about hydrogen bonding interaction?
(Multiple Choice)
4.7/5  (37)
(37)
The molecule CH4 has a tetrahedral shape. The angle between the bonds in CH4 is approximately ___.
(Multiple Choice)
4.8/5  (28)
(28)
Dioxygen difluoride whose actual three dimensional shape is provided below is non-polar.
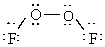
(True/False)
4.8/5  (45)
(45)
Organic compound must contain which of the following atoms?
(Multiple Choice)
4.9/5  (33)
(33)
Sketch the line-bond structure for each molecule or polyatomic ion of the following. Determine the molecular geometry and molecular polarity. (Indicate its shape and whether it is polar or nonpolar).
-H2S
A) Lewis Structure
B) Shape
C) Polar or nonpolar?
(Essay)
5.0/5  (38)
(38)
Showing 1 - 20 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)