Deck 13: Mixtures at the Molecular Level: Properties of Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/133
Play
Full screen (f)
Deck 13: Mixtures at the Molecular Level: Properties of Solutions
1
Wax is a solid mixture of hydrocarbon compounds consisting of molecules with long chains of carbon atoms. Which solvent would you expect to be most capable of dissolving wax?
A)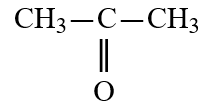
B)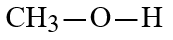
C)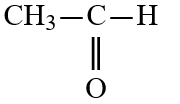
D)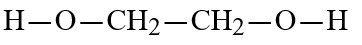
E)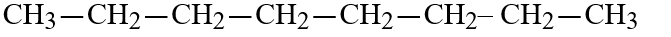
A)
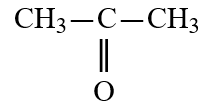
B)
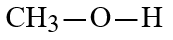
C)
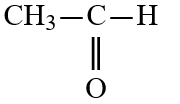
D)
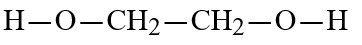
E)
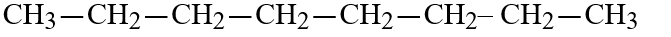
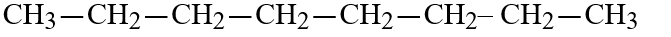
2
Wax is a solid mixture of hydrocarbon compounds consisting of molecules with long chains of carbon atoms. Which solvent would you expect to be most capable of dissolving wax?
A)H-O-H
B)CH3-O-H
C)CF3-O-H
D)H-O-CH2-CH2-O-H
E)CH3-CH2-CH2-CH2-CH2-H2-CH2-CH3
A)H-O-H
B)CH3-O-H
C)CF3-O-H
D)H-O-CH2-CH2-O-H
E)CH3-CH2-CH2-CH2-CH2-H2-CH2-CH3
CH3-CH2-CH2-CH2-CH2-H2-CH2-CH3
3
Which response lists all the following pairs that are miscible liquids?
Pair 1. octane (C8H18)and water
Pair 2. ammonia (NH3)and water
Pair 3. octane (C8H18)and carbon tetrachloride (CCl4)
A)1, 3
B)1, 2
C)2
D)2, 3
E)3
Pair 1. octane (C8H18)and water
Pair 2. ammonia (NH3)and water
Pair 3. octane (C8H18)and carbon tetrachloride (CCl4)
A)1, 3
B)1, 2
C)2
D)2, 3
E)3
2, 3
4
Concerning the process of separating of a solid substance into its component units (molecules or ions),
A)the process is exothermic and the potential energy increases.
B)the process is exothermic and the potential energy decreases.
C)the process is endothermic and the potential energy increases.
D)the process is endothermic and the potential energy decreases.
E)the process is endothermic and occurs with no change in potential energy.
A)the process is exothermic and the potential energy increases.
B)the process is exothermic and the potential energy decreases.
C)the process is endothermic and the potential energy increases.
D)the process is endothermic and the potential energy decreases.
E)the process is endothermic and occurs with no change in potential energy.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
5
Concerning the solvation step of the solution process,
A)the process is exothermic and the potential energy increases.
B)the process is exothermic and the potential energy decreases.
C)the process is endothermic and the potential energy increases.
D)the process is endothermic and the potential energy decreases.
E)the process is endothermic and occurs with no change in potential energy.
A)the process is exothermic and the potential energy increases.
B)the process is exothermic and the potential energy decreases.
C)the process is endothermic and the potential energy increases.
D)the process is endothermic and the potential energy decreases.
E)the process is endothermic and occurs with no change in potential energy.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
6
KBr has a lattice energy of -682 kJ/mol and a hydration energy of -657 kJ/mol. Using this information, what is the heat of solution, Hsol, for KBr?Hint: What happens to the crystal lattice when an ionic compound dissolves?
A)-1339 kJ/mol
B)+1339 kJ/mol
C)+25 kJ/mol
D)-657 kJ/mol
E)+657 kJ/mol
A)-1339 kJ/mol
B)+1339 kJ/mol
C)+25 kJ/mol
D)-657 kJ/mol
E)+657 kJ/mol

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
7
A solution in a beaker has some undissolved solute lying on the bottom of the beaker. If the rate of crystallization exceeds the rate of dissolution of the excess solute, the solution is described as
A)dilute.
B)concentrated.
C)unsaturated.
D)saturated.
E)supersaturated.
A)dilute.
B)concentrated.
C)unsaturated.
D)saturated.
E)supersaturated.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
8
A solution in a beaker has some un-dissolved solute lying on the bottom of the beaker. If the rate of crystallization is equal to the rate of dissolution of the excess solute, the solution is described as
A)dilute.
B)concentrated.
C)unsaturated.
D)saturated.
E)supersaturated.
A)dilute.
B)concentrated.
C)unsaturated.
D)saturated.
E)supersaturated.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
9
A solution in a beaker has some un-dissolved solute lying on the bottom of the beaker. If the rate of dissolution of the solute exceeds the rate of crystallization, the solution is described as
A)dilute.
B)concentrated.
C)unsaturated.
D)saturated.
E)supersaturated.
A)dilute.
B)concentrated.
C)unsaturated.
D)saturated.
E)supersaturated.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
10
A solution is sitting undisturbed on a side shelf in the laboratory. A small crystal of the same solute of which the solution is made was gently dropped into the solution. Suddenly, a mass of crystals forms and settles to the bottom of the container. The solution is, or must have been
A)dilute.
B)concentrated.
C)unsaturated.
D)saturated.
E)supersaturated.
A)dilute.
B)concentrated.
C)unsaturated.
D)saturated.
E)supersaturated.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following will always cause an increase in the solubility of a gas in a solvent in which the gas does not react with the solvent to form a new substance?
A)increasing the temperature of the solvent and simultaneously decreasing the pressure of the gas in the space above the solvent
B)decreasing the temperature of the solvent and simultaneously increasing the pressure of the gas in the space above the solvent
C)increasing the temperature of the solvent and simultaneously increasing the pressure of the gas in the space above the solvent
D)decreasing the temperature of the solvent and simultaneously decreasing the pressure of the gas in the space above the solvent
E)increasing the temperature of the solvent while maintaining the pressure of the gas in the space above the solvent at a set value
A)increasing the temperature of the solvent and simultaneously decreasing the pressure of the gas in the space above the solvent
B)decreasing the temperature of the solvent and simultaneously increasing the pressure of the gas in the space above the solvent
C)increasing the temperature of the solvent and simultaneously increasing the pressure of the gas in the space above the solvent
D)decreasing the temperature of the solvent and simultaneously decreasing the pressure of the gas in the space above the solvent
E)increasing the temperature of the solvent while maintaining the pressure of the gas in the space above the solvent at a set value

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
12
The solubility of O2 in water is approximately 0.00380 g L-1 when the temperature is 25.0°C and the partial pressure of gaseous oxygen is 760. torr. What will the solubility of oxygen be if the oxygen pressure is adjusted to 1000 torr?
A)0.00289 g L-1
B)0.00500 g L-1
C)1.49 g L-1
D)2.89 × 103 g L-1
E)3.46 × 103 g L-1
A)0.00289 g L-1
B)0.00500 g L-1
C)1.49 g L-1
D)2.89 × 103 g L-1
E)3.46 × 103 g L-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
13
The solubility of O2 in water is approximately 0.00380 g L-1 of water when the temperature is 25.0°C and the partial pressure of gaseous oxygen is 760 torr. The oxygen gas above the water is replaced by air at the same temperature and pressure, in which the mole fraction of oxygen is 0.210. What will the solubility of oxygen in water be under these new conditions?
A)1.05 × 10-6 g L-1
B)7.98 × 10-4 g L-1
C)1.33 × 10-3 g L-1
D)0.606 g L-1
E)1.01 g L-1
A)1.05 × 10-6 g L-1
B)7.98 × 10-4 g L-1
C)1.33 × 10-3 g L-1
D)0.606 g L-1
E)1.01 g L-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
14
A solution is made by mixing 138.2 grams of ethanol, C2H6O, (46.069 g mol-1); 103.6 grams of water (18.015 g mol-1), and 80.11 grams of methanol, CH4O, (32.042 g mol-1). What is the mole fraction of methanol in the mixture?
A)0.02504
B)0.2222
C)0.2493
D)0.3333
E)0.4490
A)0.02504
B)0.2222
C)0.2493
D)0.3333
E)0.4490

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
15
The CO2 gas sealed inside a carbonated beverage bottle has a pressure of 3.750 atm. At this pressure, the solubility of CO2 in water is 0.65 g CO2/100 g H2O. If the bottle is opened, as the gas in the space above the liquid escapes, the partial pressure of the CO2 falls to 0.30 torr, the value in the surrounding atmosphere of the room. What is the solubility of CO2 in the beverage at this new pressure?Hint: Be sure check your pressure units.
A)5.2 × 10-2 g/100 g H2O
B)5.0 × 10-4 g/100 g H2O
C)6.7 × 10-5 g/100 g H2O
D)6.8 × 10-5 g/100 g H2O
E)9.6 × 10-4 g/100 g H2O
A)5.2 × 10-2 g/100 g H2O
B)5.0 × 10-4 g/100 g H2O
C)6.7 × 10-5 g/100 g H2O
D)6.8 × 10-5 g/100 g H2O
E)9.6 × 10-4 g/100 g H2O

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
16
A solution of sodium nitrite is prepared by mixing 3.25 g of NaNO2 with 12.0 g of water. The percent, by mass, of NaNO2 is
A)28.0%.
B)23.3%.
C)27.0%.
D)21.3%.
E)37.1%.
A)28.0%.
B)23.3%.
C)27.0%.
D)21.3%.
E)37.1%.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
17
A solution of lithium hydroxide is prepared by mixing 2.00 g of LiOH with 10.0 g of water. The percent, by mass, of LiOH is
A)10.7%.
B)12.0%.
C)16.7%.
D)20.0%.
E)80.0%.
A)10.7%.
B)12.0%.
C)16.7%.
D)20.0%.
E)80.0%.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
18
How many grams of NaC2H3O2 should be dissolved in 400.0 g of water to prepare a solution that is 11.28% NaC2H3O2 by mass?
A)3.146 g
B)7.558 g
C)21.17 g
D)50.86 g
E)127.15 g
A)3.146 g
B)7.558 g
C)21.17 g
D)50.86 g
E)127.15 g

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
19
A solution of potassium nitrate is prepared by mixing 3.50 g of KNO3 with 12.0 g of water. The percent, by mass, of KNO3 is
A)22.6%.
B)23.3%.
C)28.0%.
D)29.2%.
E)41.8%.
A)22.6%.
B)23.3%.
C)28.0%.
D)29.2%.
E)41.8%.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
20
A glucose solution is prepared by dissolving 5.10 g of glucose, C6H12O6, in 110.5 g of water. What is the molality of the glucose solution?
A)0.283 m
B)0.000256 m
C)0.245 m
D)0.256 m
E)0.351 m
A)0.283 m
B)0.000256 m
C)0.245 m
D)0.256 m
E)0.351 m

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
21
An aqueous solution of orthophosphoric acid, H3PO4, has a measured density of 1.2089 g mL-1 and is 5.257 molal. How many moles of H3PO4 are there in one liter of this solution?
A)0.4261 moles
B)4.194 moles
C)4.349 moles
D)5.152 moles
E)6.355 moles
A)0.4261 moles
B)4.194 moles
C)4.349 moles
D)5.152 moles
E)6.355 moles

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
22
An aqueous solution of ethanol, C2H5OH, is 19.00% ethanol by mass and has a density of 0.9700 g mL-1. Calculate the molality of the ethanol solution.
A)4.000 m
B)4.124 m
C)4.252 m
D)5.092 m
E)14.48 m
A)4.000 m
B)4.124 m
C)4.252 m
D)5.092 m
E)14.48 m

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
23
An aqueous solution of glycerol, C3H8O3, is 48.0% glycerol by mass and has a density of 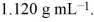 Calculate the molality of the glycerol solution.
Calculate the molality of the glycerol solution.
A)11.2 m
B)5.84 m
C)0.584 m
D)0.521 m
E)10.0 m
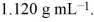 Calculate the molality of the glycerol solution.
Calculate the molality of the glycerol solution.A)11.2 m
B)5.84 m
C)0.584 m
D)0.521 m
E)10.0 m

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
24
A solution is prepared by mixing 0.3355 moles of NaNO3 (84.995 g mol-1)with 235.0 g of water (18.015 g mol-1). Its density is 1.0733 g mL-1. What is the percent by mass of NaNO3 in the solution?
A)10.16%
B)10.82%
C)11.61%
D)14.19%
E)26.56%
A)10.16%
B)10.82%
C)11.61%
D)14.19%
E)26.56%

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
25
A solution is prepared by mixing 0.3355 moles of NaNO3 (84.995 g mol-1)with 235.0 g of water (18.015 g mol-1). Its density is 1.0733 g mL-1. What is the molality of the solution?
A)0.6474 m
B)0.7004 m
C)1.320 m
D)1.428 m
E)1.545 m
A)0.6474 m
B)0.7004 m
C)1.320 m
D)1.428 m
E)1.545 m

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
26
An aqueous solution is prepared by mixing 0.2750 moles of NaOH (40.00 g mol-1)with 189.0 g of water. Its density is 1.065 g mL-1. What is the percent by weight of NaOH in the solution?
A)0.001375%
B)5.500%
C)5.858%
D)13.75%
E)20.00%
A)0.001375%
B)5.500%
C)5.858%
D)13.75%
E)20.00%

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
27
An aqueous solution of nitric acid has a density of 1.084 g mL-1 and a measured concentration of 2.580 molar. What is the percent by weight of nitric acid in the solution?
A)2.380%
B)13.82%
C)15.00%
D)22.29%
E)44.38%
A)2.380%
B)13.82%
C)15.00%
D)22.29%
E)44.38%

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
28
Which is a concentration unit whose value changes if the temperature of an aqueous solution is changed?
A)mole fraction
B)molarity
C)molality
D)mass fraction
E)percent by weight
A)mole fraction
B)molarity
C)molality
D)mass fraction
E)percent by weight

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
29
Consider a 0.900 M Al(NO3)3 solution. This solution has a nitrate ion concentration of
A)0.300 M.
B)0.900 M.
C)2.70 M.
D)3.60 M.
E)8.10 M.
A)0.300 M.
B)0.900 M.
C)2.70 M.
D)3.60 M.
E)8.10 M.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
30
A solution of ethylene glycol (C2H6O2)in water is 3.981 molar and has a density of 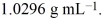 Calculate the percent, by weight, of ethylene glycol in the solution.
Calculate the percent, by weight, of ethylene glycol in the solution.
A)3.867%
B)4.099%
C)15.14%
D)24.00%
E)25.45%
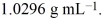 Calculate the percent, by weight, of ethylene glycol in the solution.
Calculate the percent, by weight, of ethylene glycol in the solution.A)3.867%
B)4.099%
C)15.14%
D)24.00%
E)25.45%

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
31
A solution of sodium nitrate, NaNO3, in water is 5.181 molar and its density is 1.2680 g mL-1. Calculate the percent, by weight, of sodium nitrate in the solution.
A)7.939%
B)17.21%
C)24.47%
D)29.56%
E)34.73%
A)7.939%
B)17.21%
C)24.47%
D)29.56%
E)34.73%

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
32
An aqueous solution of ethanol, C2H5OH, is 19.00% ethanol by mass and has a density of 0.9700 g mL-1. Calculate the molarity of the ethanol solution.
A)4.001 M
B)4.124 M
C)4.252 M
D)5.092 M
E)14.48 M
A)4.001 M
B)4.124 M
C)4.252 M
D)5.092 M
E)14.48 M

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
33
An aqueous solution of glycerol, C3H8O3, is 48.0% glycerol by mass andhas a density of 1.120 g mL-1. Calculate the molarity of the glycerol solution.
A)12.2 M
B)5.84 M
C)0.584 M
D)0.521 M
E)0.465 M
A)12.2 M
B)5.84 M
C)0.584 M
D)0.521 M
E)0.465 M

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
34
A solution is prepared by mixing 0.3355 moles of NaNO3 (84.995 g mol-1)with 235.0 g of water (18.015 g mol-1). Its density is 1.0733 g mL-1. What is the molarity of the solution?
A)1.186 M
B)1.273 M
C)1.350 M
D)1.366 M
E)1.428 M
A)1.186 M
B)1.273 M
C)1.350 M
D)1.366 M
E)1.428 M

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
35
An aqueous solution of glycerol, C3H8O3, in which the mole fraction of C3H8O3 is 0.07070 was found to have a density of 1.0350 g mL-1. What is the molarity of this solution?Hint: Assume one mole of glycerol and solve for moles of water. From there you can find the mass and then volume of the solution.
A)3.147 molar
B)4.371 molar
C)4.223 molar
D)2.938 molar
E)3.651 molar
A)3.147 molar
B)4.371 molar
C)4.223 molar
D)2.938 molar
E)3.651 molar

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
36
An aqueous solution of glycerol, C3H8O3 is prepared by adding 150.0 g of glycerol to 200.0 g of water. What is the mole fraction of glycerol in the final solution?
A)0.571
B)0.429
C)0.750
D)0.872
E)0.128
A)0.571
B)0.429
C)0.750
D)0.872
E)0.128

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
37
An aqueous solution of ethanol, C2H5OH is prepared by adding 200. g of ethanol to 50.0 g of water. What is the mole fraction of ethanol in the final solution?
A)0.200
B)0.610
C)0.390
D)0.250
E)0.800
A)0.200
B)0.610
C)0.390
D)0.250
E)0.800

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
38
The vapor pressure of a solution containing a nonvolatile solute is directly proportional to the
A)mole fraction of the solvent.
B)mole fraction of the solute.
C)molality of the solvent.
D)molarity of the solvent.
E)osmotic pressure of the solute.
A)mole fraction of the solvent.
B)mole fraction of the solute.
C)molality of the solvent.
D)molarity of the solvent.
E)osmotic pressure of the solute.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
39
When a nonvolatile solute such as ammonium sulfate is dissolved in a solvent like water, one of the observed effects is
A)a decrease in the vapor pressure of the solvent.
B)an increase in the vapor pressure of the solute.
C)an increase in the freezing point of the liquid.
D)a decrease in the boiling point of the liquid.
E)scattering of light beams by the solute particles in the solution.
A)a decrease in the vapor pressure of the solvent.
B)an increase in the vapor pressure of the solute.
C)an increase in the freezing point of the liquid.
D)a decrease in the boiling point of the liquid.
E)scattering of light beams by the solute particles in the solution.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
40
At 23.0°C, the vapor pressure of acetonitrile, CH3CN, is 81.0 torr while that of acetone, C3H6O, is 184.5 torr. What is the vapor pressure of a solution that contains 0.550 moles of acetonitrile and 0.350 moles of acetone? (Assume the mixture behaves as an ideal solution.)
A)109 torr
B)121 torr
C)130 torr
D)144 torr
E)239 torr
A)109 torr
B)121 torr
C)130 torr
D)144 torr
E)239 torr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
41
During osmosis,
A)pure solvent passes through a membrane but solutes do not.
B)pure solute passes through a membrane but solvent does not.
C)pure solvent moves in one direction through the membrane while the solution moves through the membrane in the other direction.
D)pure solvent moves in one direction through the membrane while the solute moves through the membrane in the other direction.
E)pure solute moves in one direction through the membrane while the solution moves through the membrane in the other direction.
A)pure solvent passes through a membrane but solutes do not.
B)pure solute passes through a membrane but solvent does not.
C)pure solvent moves in one direction through the membrane while the solution moves through the membrane in the other direction.
D)pure solvent moves in one direction through the membrane while the solute moves through the membrane in the other direction.
E)pure solute moves in one direction through the membrane while the solution moves through the membrane in the other direction.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
42
A very dilute solution contains 116 mg of fructose (molar mass = 180.16 g mol-1)in 1.000 liter of solution. It is placed in an osmotic membrane bladder, which is then suspended in pure water. What osmotic pressure would develop across the membrane if the temperature is 26.0°C?
A)3.36 torr
B)12.0 torr
C)151 torr
D)475 torr
E)1217 torr
A)3.36 torr
B)12.0 torr
C)151 torr
D)475 torr
E)1217 torr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
43
Which aqueous solution will have the highest boiling point temperature?
A)0.100 molal NiBr2(aq)
B)0.100 molal MgSO4(aq)
C)0.150 molal NH4NO3(aq)
D)0.150 molal Na2SO4(aq)
E)0.250 molal CH3OH(aq)
A)0.100 molal NiBr2(aq)
B)0.100 molal MgSO4(aq)
C)0.150 molal NH4NO3(aq)
D)0.150 molal Na2SO4(aq)
E)0.250 molal CH3OH(aq)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
44
Which aqueous solution will have the lowest boiling point temperature?
A)0.100 molal NiBr2(aq)
B)0.200 molal MgSO4(aq)
C)0.150 molal NH4NO3(aq)
D)0.150 molal Na2SO4(aq)
E)0.250 molal CH3OH(aq)
A)0.100 molal NiBr2(aq)
B)0.200 molal MgSO4(aq)
C)0.150 molal NH4NO3(aq)
D)0.150 molal Na2SO4(aq)
E)0.250 molal CH3OH(aq)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
45
Which solution has the highest osmotic pressure at 25°C?
A)0.200 M (NH4)2SO4
B)0.200 M KClO3
C)0.200 M C2H6O, ethanol
D)0.200 M NiF2
E)0.200 M Fe(NO3)3
A)0.200 M (NH4)2SO4
B)0.200 M KClO3
C)0.200 M C2H6O, ethanol
D)0.200 M NiF2
E)0.200 M Fe(NO3)3

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
46
Arrange these aqueous solutions in order of increasing boiling points: 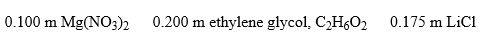
A)C2H6O2 < Mg(NO3)2 < LiCl
B)Mg(NO3)2 < LiCl < C2H6O2
C)C2H6O2 < LiCl < Mg(NO3)2
D)LiCl < C2H6O2 < Mg(NO3)2
E)Mg(NO3)2 < C2H6O2 < LiCl
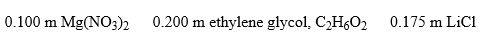
A)C2H6O2 < Mg(NO3)2 < LiCl
B)Mg(NO3)2 < LiCl < C2H6O2
C)C2H6O2 < LiCl < Mg(NO3)2
D)LiCl < C2H6O2 < Mg(NO3)2
E)Mg(NO3)2 < C2H6O2 < LiCl

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
47
A dilute aqueous solution of CaCl2 contains 0.159 grams of solute per liter of solution. It is fully dissociated. What is its osmotic pressure at 20.0°C?Hint: CaCl2 is an ionic compound.
A)1.79 torr
B)3.49 torr
C)26.2 torr
D)78.6 torr
E)2.65 × 103 torr
A)1.79 torr
B)3.49 torr
C)26.2 torr
D)78.6 torr
E)2.65 × 103 torr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
48
At 28.0°C, the vapor pressure of n-propyl mercaptan, C3H7SH, is 175 torr, while that of acetonitrile, CH3CN, is 102 torr. What is the vapor pressure, at 28.0°C, of a solution made by mixing 100.0 g of C3H7SH and 100.0 g CH3CN, if Raoult's Law is obeyed?
A)35.7 torr
B)128 torr
C)139 torr
D)149 torr
E)277 torr
A)35.7 torr
B)128 torr
C)139 torr
D)149 torr
E)277 torr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
49
A molecular solute with a molar mass of 50.0 g mol-1 is dissolved in 500 g of water and the resulting solution has a boiling point of 101.53°C. How many grams of solute were in the solution Kb = 0.512 °C m-1
A)30.0 grams
B)75.0 grams
C)100 grams
D)125 grams
E)150 grams
A)30.0 grams
B)75.0 grams
C)100 grams
D)125 grams
E)150 grams

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
50
A solution contains 221 g of glycerol (C3H8O3)in 600 grams of water. For the solvent, the Kf is 1.86 °C m-1 and Kb is 0.512 °C m-1. What should the boiling point of the solution be?
A)100.02°C
B)2.04°C
C)101.65°C
D)102.04°C
E)3.62°C
A)100.02°C
B)2.04°C
C)101.65°C
D)102.04°C
E)3.62°C

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
51
A solution, which was made by dissolving 62.07 g of a nonelectrolyte in 500 g of water, exhibits a freezing point of -1.86°C. What is the molecular weight of this nonelectrolyte compound? For water, Kf is 1.86°C m-1 and Kb is 0.512°C m-1.
A)57.7 g mol-1
B)62.07 g mol-1
C)115 g mol-1
D)124 g mol-1
E)231 g mol-1
A)57.7 g mol-1
B)62.07 g mol-1
C)115 g mol-1
D)124 g mol-1
E)231 g mol-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
52
How many moles of the nonelectrolyte, propylene glycol (C3H8O2)should be dissolved in 800.0 g of water to prepare a solution whose freezing point is -3.72°C?For water, Kf is 1.86°C m-1 and Kb is 0.512°C m-1.
A)1.60 moles
B)2.00 moles
C)2.50 moles
D)2.98 moles
E)4.65 moles
A)1.60 moles
B)2.00 moles
C)2.50 moles
D)2.98 moles
E)4.65 moles

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
53
Which can be used to calculate the osmotic pressure of a solution?
A)de Broglie equation
B)Tyndall factor
C)Peters equation
D)van der Waals equation
E)van't Hoff equation
A)de Broglie equation
B)Tyndall factor
C)Peters equation
D)van der Waals equation
E)van't Hoff equation

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
54
How many grams of glycerol (C3H8O3, a nonelectrolyte)should be dissolved in 600 g of water to prepare a solution whose freezing point is -4.65°C? For water, Kf is 1.86°C m-1 and Kb is 0.512 °C m-1.
A)22.1 grams
B)93.6 grams
C)138 grams
D)384 grams
E)478 grams
A)22.1 grams
B)93.6 grams
C)138 grams
D)384 grams
E)478 grams

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
55
Which aqueous solution has the highest boiling point?
A)0.200 m KCl(aq)
B)0.200 m Na2SO4(aq)
C)0.200 m Ca(NO3)2(aq)
D)0.200 m C3H8O3 (aq), glycerol
E)0.200 m Na3PO4(aq)
A)0.200 m KCl(aq)
B)0.200 m Na2SO4(aq)
C)0.200 m Ca(NO3)2(aq)
D)0.200 m C3H8O3 (aq), glycerol
E)0.200 m Na3PO4(aq)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
56
A solution is made by dissolving 0.840 moles of sodium hydroxide in 300.0 g of water. If the van't Hoff factor, i, for this particular concentration is 1.70, what is the expected freezing point of this solution? Kf = 1.86 °C m-1.
A)+0.80°C
B)-2.66°C
C)+3.06°C
D)-8.85°C
E)-9.97°C
A)+0.80°C
B)-2.66°C
C)+3.06°C
D)-8.85°C
E)-9.97°C

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
57
9.92 grams of a compound with a molar mass of 124.0 grams/mole when dissolved in 150.0 grams of water gave a solution that had a freezing point of -2.69°C. Calculate the experimental value of the van't Hoff factor. Kf = 1.86 °C m-1.
A)1.21
B)1.45
C)1.54
D)2.69
E)2.71
A)1.21
B)1.45
C)1.54
D)2.69
E)2.71

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
58
A solution is made by dissolving 1.25 moles of magnesium nitrate in 300.0 g of water. If the van't Hoff factor, i, for this particular concentration is 2.25, what is the expected freezing point of this solution? Kf = 1.86 °C m-1.
A)-0.45°C
B)-1.57°C
C)-3.44°C
D)-5.23°C
E)-17.4°C
A)-0.45°C
B)-1.57°C
C)-3.44°C
D)-5.23°C
E)-17.4°C

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
59
An aqueous solution which is 12.00% sodium hydroxide by weight has a freezing point of ?10.40°C. What is the observed value of the van't Hoff factor, i, in this solution? Kf = 1.86 °C m-1.
A)1.59
B)1.86
C)1.64
D)1.75
E)1.70
A)1.59
B)1.86
C)1.64
D)1.75
E)1.70

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
60
Which aqueous solution should have the highest osmotic pressure?
A)0.100 molar Al(NO3)3
B)0.150 molar Ba(NO3)2
C)0.100 molar CaCl2
D)0.150 molar NaCl
E)0.200 molar NH3
A)0.100 molar Al(NO3)3
B)0.150 molar Ba(NO3)2
C)0.100 molar CaCl2
D)0.150 molar NaCl
E)0.200 molar NH3

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
61
Below is a diagram of vapor pressure versus composition for a mixture of two liquids. The dark straight lines represent the vapor pressure of each pure liquid, while the gray curved line gives the vapor pressure of the mixture. Using this information, which of the following statements is true about the mixture? 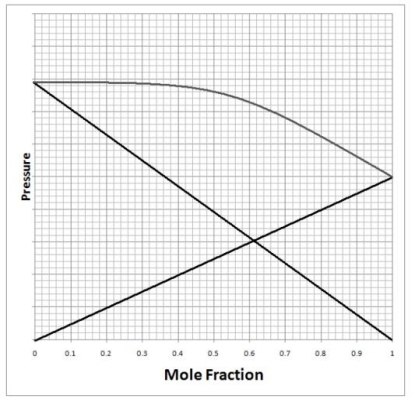
A)The mixture is an ideal mixture that has no deviation from Raoult's Law.
B)The mixture is an ideal mixture with a positive deviation from Raoult's Law.
C)The mixture is not ideal and interactions between unlike molecules in solution are weaker than the interactions between like molecules in pure solution.
D)The mixture is not ideal and interactions between unlike molecules in solution are stronger than the interactions between like molecules in the pure solution.
E)The mixture is ideal and interactions between unlike molecules in solution are the same as the interactions between like molecules in the pure solution.

A)The mixture is an ideal mixture that has no deviation from Raoult's Law.
B)The mixture is an ideal mixture with a positive deviation from Raoult's Law.
C)The mixture is not ideal and interactions between unlike molecules in solution are weaker than the interactions between like molecules in pure solution.
D)The mixture is not ideal and interactions between unlike molecules in solution are stronger than the interactions between like molecules in the pure solution.
E)The mixture is ideal and interactions between unlike molecules in solution are the same as the interactions between like molecules in the pure solution.

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
62
At 28.0°C, the vapor pressure of npropyl mercaptan, C3H7SH, is 175 torr, while that of acetonitrile, CH3CN, is 102 torr. What is the vapor pressure, at 28.0°C, of a solution made by mixing 120.0 g of C3H7SH and 80.0 g CH3CN, if Raoult's Law is obeyed?Hint: Organizing the given information into moles and mole fraction of each component will help in solving this problem.
A)131 torr
B)135 torr
C)142 torr
D)146 torr
E)277 torr
A)131 torr
B)135 torr
C)142 torr
D)146 torr
E)277 torr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
63
At 28.0°C, the vapor pressure of n-propyl mercaptan, C3H7SH, is 175 torr, while that ofacetonitrile, CH3CN, is 102 torr. What is the vapor pressure, at 28.0°C, of a solution made bymixing 80.0 g of C3H7SH and 120.0 g CH3CN, if Raoult's Law is obeyed?Hint: Organizing the given information into moles and mole fraction of each component will help in solving this problem.
A)121 torr
B)131 torr
C)139 torr
D)146 torr
E)156 torr
A)121 torr
B)131 torr
C)139 torr
D)146 torr
E)156 torr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
64
At 28.0°C, the vapor pressure of acetonitrile, CH3CN, is 102.0 torr while that of acetone, C3H6O, is 228.9 torr, and for CS2 the value is 378.7 torr. A three-component solution is made by adding 0.300 moles of CH3CN and 0.400 moles of C3H6O to 0.350 moles of CS2. The mixture behaves as an ideal mixture. What is the vapor pressure of the solution?Hint: Organizing the given information into moles and mole fraction of each component will help in solving this problem.
A)203 torr
B)385 torr
C)262 torr
D)275 torr
E)610 torr
A)203 torr
B)385 torr
C)262 torr
D)275 torr
E)610 torr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
65
At 28.0°C, the vapor pressure of pure carbon disulfide, CS2, is 378.7 torr, while that of acetone, C3H6O, is 228.9 torr. What is the vapor pressure, at 28.0°C, of a solution made by mixing 0.250 moles of carbon disulfide and 0.450 moles of acetone, if Raoult's Law is obeyed?Hint: Organizing the given information into moles and mole fraction of each component will help in solving this problem.
A)198 torr
B)228 torr
C)282 torr
D)325 torr
E)425 torr
A)198 torr
B)228 torr
C)282 torr
D)325 torr
E)425 torr

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
66
What is the expected freezing point of a solution that contains 25.0 g of fructose, C6H12O6, in 250.0 g of H2O? For water, Kf = 1.86 °C m-1.
A)-0.10°C
B)+0.10°C
C)-0.186°C
D)+0.186°C
E)-1.03°C
A)-0.10°C
B)+0.10°C
C)-0.186°C
D)+0.186°C
E)-1.03°C

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
67
What is the freezing point of a solution that contains 20.0 g of glucose (C6H12O6)in 100.0 g of H2O? Kf for water is 1.86°C/m.
A)-0.206°C
B)-2.06°C
C)-1.86°C
D)+0.111°C
E)+1.11°C
A)-0.206°C
B)-2.06°C
C)-1.86°C
D)+0.111°C
E)+1.11°C

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
68
What is the normal boiling point of a solution that contains 30.0 g of glucose (C6H12O6)in 100.0 g of H2O? Kb for water is 0.512°C/m.
A)0.17°C
B)99.01°C
C)101.67°C
D)100.85°C
E)101.53°C
A)0.17°C
B)99.01°C
C)101.67°C
D)100.85°C
E)101.53°C

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
69
Pure benzene, C6H6, has a freezing point of 5.45°C. Its freezing point depressionconstant is: Kf = 5.07 °C m-1. A solution was made by taking 24.20 g of an unknown nonelectrolyte and dissolving it in 125.0 g of benzene. The measured freezing point of the solution was -1.65°C. Calculate the molecular weight of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
A)138 g mol-1
B)145 g mol-1
C)258 g mol-1
D)272 g mol-1
E)595 g mol-1
A)138 g mol-1
B)145 g mol-1
C)258 g mol-1
D)272 g mol-1
E)595 g mol-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
70
Pure cyclohexane, C6H12, has a freezing point of 6.53°C. Its freezing point depression constant is: Kf = 20.0 °C m-1. A solution was made by taking 11.40 g of an unknown nonelectrolyte and dissolving it in 150.0 g of cyclohexane. The measured freezing point of the solution was -0.78°C. Calculate the molecular weight of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
A)27.8 g mol-1
B)46.8 g mol-1
C)208 g mol-1
D)264 g mol-1
E)1949 g mol-1
A)27.8 g mol-1
B)46.8 g mol-1
C)208 g mol-1
D)264 g mol-1
E)1949 g mol-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
71
Pure cyclohexane, C6H12, has a freezing point of 6.53°C. Its freezing point depression constant is: Kf = 20.0 °C m-1. A solution was made by taking 18.55 g of an unknown nonelectrolyte and dissolving it in 150.0 g of cyclohexane. The measured freezing point of the solution was -4.28°C. Calculate the molecular weight of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
A)61.8 g mol-1
B)66.8 g mol-1
C)229 g mol-1
D)578 g mol-1
E)1099 g mol-1
A)61.8 g mol-1
B)66.8 g mol-1
C)229 g mol-1
D)578 g mol-1
E)1099 g mol-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
72
Pure glacial acetic acid, HC2H3O2, has a freezing point of 16.62°C. Its freezing point depression constant is: Kf = 3.57 °C m-1. A solution was made by taking 9.755 g of an unknown nonelectrolyte and dissolving it in 90.50 g of glacial acetic acid. The measured freezing point of the solution was 8.64°C. Calculate the molecular weight of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
A)24.4 g mol-1
B)30.5 g mol-1
C)45.3 g mol-1
D)48.2 g mol-1
E)174 g mol-1
A)24.4 g mol-1
B)30.5 g mol-1
C)45.3 g mol-1
D)48.2 g mol-1
E)174 g mol-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
73
Pure benzene, C6H6, has a freezing point of 5.45°C. Its freezing point depressionconstant is: Kf = 5.07 °C m-1. A solution was made by taking 10.00 g of an unknown nonelectrolyte and dissolving it in 105.0 g of benzene. The measured freezing point of the solution was -1.05°C. Calculate the molecular weight of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
A)74.3 g mol-1
B)82.7 g mol-1
C)110 g mol-1
D)122 g mol-1
E)460 g mol-1
A)74.3 g mol-1
B)82.7 g mol-1
C)110 g mol-1
D)122 g mol-1
E)460 g mol-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
74
An aqueous ethylene glycol solution being considered for use as a radiator coolant is 16.0% C2H6O2 by weight. What would be the expected boiling point of this solution? For water, Kf is 1.86 °C m-1 and Kb = 0.512 °C m?1.Hint: convert percent by mass to molality, then find T.
A)100.3°C
B)101.6°C
C)105.1°C
D)106.0°C
E)156.5°C
A)100.3°C
B)101.6°C
C)105.1°C
D)106.0°C
E)156.5°C

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
75
Pure chloroform, CHCl3, has a boiling point of 61.23°C. Its boiling point elevation constant, Kb, is 3.63°C m-1. A solution was made by taking 11.25 g of an unknown nonelectrolyte and dissolving it in 115.5 g of chloroform. The measured boiling point of the solution was 65.46°C. Calculate the molecular weight of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
A)83.6 g mol-1
B)113 g mol-1
C)114 g mol-1
D)120 g mol-1
E)158 g mol-1
A)83.6 g mol-1
B)113 g mol-1
C)114 g mol-1
D)120 g mol-1
E)158 g mol-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
76
Which property of a solution is not a colligative property?
A)solubility of a solute
B)freezing point depression
C)boiling point elevation
D)osmotic pressure
E)vapor pressure lowering
A)solubility of a solute
B)freezing point depression
C)boiling point elevation
D)osmotic pressure
E)vapor pressure lowering

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
77
An aqueous solution made by dissolving 168 mg of an unknown compound (a nonelectrolyte)in enough water to make 500.0 mL of solution, develops an osmotic pressure of 5.22 torr at a temperature of 23.5°C. What is the molecular mass of this unknown compound?Hint: Find molarity, then moles, then molar mass, being sure to keep track of units.
A)94.3 g mol-1
B)124 g mol-1
C)943 g mol-1
D)1.19 × 103 g mol-1
E)1.57 × 103 g mol-1
A)94.3 g mol-1
B)124 g mol-1
C)943 g mol-1
D)1.19 × 103 g mol-1
E)1.57 × 103 g mol-1

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
78
Which aqueous solution will have the lowest freezing point temperature?
A)0.100 molal NaBr(aq)
B)0.100 molal MgSO4(aq)
C)0.150 molal KClO3(aq)
D)0.150 molal MgCl2(aq)
E)0.250 molal C6H12O6(aq)
A)0.100 molal NaBr(aq)
B)0.100 molal MgSO4(aq)
C)0.150 molal KClO3(aq)
D)0.150 molal MgCl2(aq)
E)0.250 molal C6H12O6(aq)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
79
A solution is prepared by mixing 0.3355 moles of NaNO3 with 235.0 g of water. Its density is 1.0733 g mL-1. If Kf = 1.86 °C m-1 and the solute is completely ionized, what is the expected freezing point of the solution?Hint: be sure to classify the solute as an electrolyte or non-electrolyte.
A)2.65°C
B)2.87°C
C)-5.31°C
D)5.75°C
E)7.97°C
A)2.65°C
B)2.87°C
C)-5.31°C
D)5.75°C
E)7.97°C

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck
80
Which aqueous solution will have the highest freezing point temperature?Hint: be sure to closely read the question.
A)0.100 molal MgBr2(aq)
B)0.100 molal MgSO4(aq)
C)0.150 molal KClO3(aq)
D)0.150 molal MgCl2(aq)
E)0.250 molal C6H12O6(aq)
A)0.100 molal MgBr2(aq)
B)0.100 molal MgSO4(aq)
C)0.150 molal KClO3(aq)
D)0.150 molal MgCl2(aq)
E)0.250 molal C6H12O6(aq)

Unlock Deck
Unlock for access to all 133 flashcards in this deck.
Unlock Deck
k this deck


