Exam 13: Mixtures at the Molecular Level: Properties of Solutions
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
When a nonvolatile solute such as ammonium sulfate is dissolved in a solvent like water, one of the observed effects is
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
A
Wax is a solid mixture of hydrocarbon compounds consisting of molecules with long chains of carbon atoms. Which solvent would you expect to be most capable of dissolving wax?
Free
(Multiple Choice)
4.7/5  (42)
(42)
Correct Answer:
E
Many marine organisms that require oxygen live at ocean depths where high pressures and low temperatures would not support humans. Based on what you have learned in this chapter, what is one reason that organisms can survive under these conditions?
Free
(Short Answer)
4.9/5  (26)
(26)
Correct Answer:
Oxygen becomes more soluble at higher pressures and colder temperatures, both of which are present at the "bottom"of the ocean. Thus, the environment is oxygen rich. These organisms must have adaptations that allow them to survive under high temperatures and low-pressures.
Dry air is a mixture of gases in which the partial pressure of nitrogen gas at 1.000 atm is typically 0.78 atm while that of argon gas is 0.009 atm. A liquid sample was exposed to dry air at 1.000 atm and a given temperature and found to have a nitrogen gas concentration of 5.30 × 10−4 M. If argon were added to the dry air until the partial pressure of argon was now 0.400 atm, the concentration of nitrogen gas in the liquid sample would now be ________.Hint: Think of Dalton's law of partial pressures.
(Short Answer)
4.8/5  (39)
(39)
Calculate the freezing point of a solution made from 22.0 g of octane (C8H18)dissolved in 248.0 g of benzene. Benzene freezes at 5.50 °C and its Kf value is 5.07 °C/m.
(Short Answer)
4.8/5  (36)
(36)
Arrange these aqueous solutions in order of increasing boiling points: 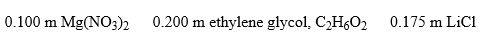
(Multiple Choice)
5.0/5  (38)
(38)
Pure cyclohexane, C6H12, has a molar mass of 84.161 g mol−1 and a density of0.7785 g mL−1, a freezing point of 6.53°C, and a boiling point of 80.72°C. Its freezing point depression and boiling point elevation constants are: Kf = 20.0 °C m−1; Kb = 2.69°C m−1. A solution was made by taking 15.46 g of an unknown nonelectrolyte and dissolving it in 125.0 g of cyclohexane. The measured freezing point of the solution was -4.28°C. Calculate the molar mass of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
(Short Answer)
4.7/5  (31)
(31)
Pure glacial acetic acid, HC2H3O2, has a molar mass of 60.052 g mol−1 and a density of 1.0492 g mL−1, a freezing point of 16.62°C, and a boiling point of 118.3°C. Its freezing point depression and boiling point elevation constants are: Kf = 3.57 °C m−1; Kb = 3.07 °C m−1. A solution was made by taking 19.51 g of an unknown nonelectrolyte and dissolving it in 181.0 g of glacial acetic acid. The measured freezing point of the solution was 8.64°C. Calculate the molar mass of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
(Short Answer)
4.9/5  (36)
(36)
What is the mole fraction of ethylene glycol, C2H6O2, in an aqueous solution that is 50.0% ethylene glycol by mass?
(Short Answer)
4.8/5  (37)
(37)
At 28.0°C, the vapor pressure of npropyl mercaptan, C3H7SH, is 175 torr, while that of acetonitrile, CH3CN, is 102 torr. What is the vapor pressure, at 28.0°C, of a solution made by mixing 120.0 g of C3H7SH and 80.0 g CH3CN, if Raoult's Law is obeyed?Hint: Organizing the given information into moles and mole fraction of each component will help in solving this problem.
(Multiple Choice)
4.9/5  (34)
(34)
An aqueous ethylene glycol solution being considered for use as a radiator coolant is 16.0% C2H6O2 by weight. What would be the expected boiling point of this solution? For water, Kf is 1.86 °C m-1 and Kb = 0.512 °C m?1.Hint: convert percent by mass to molality, then find T.
(Multiple Choice)
4.8/5  (26)
(26)
How many grams of NaC2H3O2 should be dissolved in 400.0 g of water to prepare a solution that is 11.28% NaC2H3O2 by mass?
(Multiple Choice)
4.8/5  (37)
(37)
A solution is made by mixing 138.2 grams of ethanol, C2H6O, (46.069 g mol-1); 103.6 grams of water (18.015 g mol-1), and 80.11 grams of methanol, CH4O, (32.042 g mol-1). What is the mole fraction of methanol in the mixture?
(Multiple Choice)
4.8/5  (26)
(26)
Pure chloroform, CHCl3, has a boiling point of 61.23°C. Its boiling point elevation constant, Kb, is 3.63°C m-1. A solution was made by taking 11.25 g of an unknown nonelectrolyte and dissolving it in 115.5 g of chloroform. The measured boiling point of the solution was 65.46°C. Calculate the molecular weight of the unknown substance.Hint: Find molality, then moles, then molar mass, being sure to keep track of units.
(Multiple Choice)
4.9/5  (40)
(40)
A solution contains 25.50 grams of NaNO3 (M = 84.99 g mol−1)in 250.0 grams of water. Its density is 1.0620 g mL−1. Calculate the molality of the solution.
(Short Answer)
4.9/5  (42)
(42)
An aqueous solution of nitric acid has a density of 1.084 g mL-1 and a measured concentration of 2.580 molar. What is the percent by weight of nitric acid in the solution?
(Multiple Choice)
4.7/5  (43)
(43)
An aqueous solution that would cause red blood cells to burst from lowered osmotic pressure is called a(n)________ solution.
(Multiple Choice)
4.9/5  (33)
(33)
What is the expected freezing point of a solution that contains 25.0 g of fructose, C6H12O6, in 250.0 g of H2O? For water, Kf = 1.86 °C m-1.
(Multiple Choice)
4.8/5  (44)
(44)
A solution is made by dissolving 48.07 g of MgSO4∙7H2O in 250.0 grams of water. What is the expected freezing point of this solution if the van't Hoff factor is 1.90? Kf = 1.86 °C m−1.Hint: Don't forget to include the 7H2O when calculating the molar mass of solute.
(Short Answer)
4.8/5  (36)
(36)
Showing 1 - 20 of 133
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)