Deck 19: The Transition Metals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/38
Play
Full screen (f)
Deck 19: The Transition Metals
1
What is the correct arrangement of Sc, V, and Cu in order of increasing melting point?
A) Sc, V, Cu
B) Cu, Sc, V
C) Sc, Cu, V
D) V, Sc, Cu
E) Cu, V, Sc
A) Sc, V, Cu
B) Cu, Sc, V
C) Sc, Cu, V
D) V, Sc, Cu
E) Cu, V, Sc
Cu, Sc, V
2
What is the correct arrangement of Cr, Ni, and Zn in order of decreasing density?
A) Cr, Ni, Zn
B) Ni, Cr, Zn
C) Zn, Ni, Cr
D) Zn, Cr, Ni
E) Ni, Zn, Cr
A) Cr, Ni, Zn
B) Ni, Cr, Zn
C) Zn, Ni, Cr
D) Zn, Cr, Ni
E) Ni, Zn, Cr
Cr, Ni, Zn
3
What is the correct arrangement of Pd, Ni, and Pt in order of decreasing density?
A) Pd, Ni, Pt
B) Ni, Pd, Pt
C) Pt, Ni, Pd
D) Pt, Pd, Ni
E) Ni, Pt, Pd
A) Pd, Ni, Pt
B) Ni, Pd, Pt
C) Pt, Ni, Pd
D) Pt, Pd, Ni
E) Ni, Pt, Pd
Pt, Pd, Ni
4
What is the correct arrangement of Hf, Ti, and Zr in order of increasing melting point?
A) Hf, Ti, Zr
B) Ti, Zr, Hf
C) Zr, Hf, Ti
D) Hf, Zr, Ti
E) Ti, Hf, Zr
A) Hf, Ti, Zr
B) Ti, Zr, Hf
C) Zr, Hf, Ti
D) Hf, Zr, Ti
E) Ti, Hf, Zr

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
5
The transition metals with the electron configurations of 4s23d2 and 5s24d7 are
A) Cr and Ag.
B) Zr and Au.
C) Ti and Rh.
D) Ti and Pd.
E) Sc and Rh.
A) Cr and Ag.
B) Zr and Au.
C) Ti and Rh.
D) Ti and Pd.
E) Sc and Rh.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following transition metals in the 3d series has the least variability in preferred oxidation number?
A) Mn
B) Cu
C) Cr
D) V
E) Sc
A) Mn
B) Cu
C) Cr
D) V
E) Sc

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following transition metals in the 3d series has the most variability in preferred oxidation number?
A) Mn
B) Cu
C) Cr
D) V
E) Sc
A) Mn
B) Cu
C) Cr
D) V
E) Sc

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
8
The standard reduction potential for aqueous Ag+ is 0.80 V. This value of the reduction potential
A) means silver is easily oxidized.
B) means silver is a good reducing agent.
C) means Ag+ is a good oxidizing agent.
D) is why silver forms oxy-ions.
E) means Ag is easily reduced.
A) means silver is easily oxidized.
B) means silver is a good reducing agent.
C) means Ag+ is a good oxidizing agent.
D) is why silver forms oxy-ions.
E) means Ag is easily reduced.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
9
Which transition metal compounds are most likely to found as pure elements?
A) Au, Ag, As
B) Au, Pt, Ir
C) Cu, Zn, Pt
D) Hg, Fe, Pt
E) Hg, Pt, Au
A) Au, Ag, As
B) Au, Pt, Ir
C) Cu, Zn, Pt
D) Hg, Fe, Pt
E) Hg, Pt, Au

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
10
Which is the chemical formula for the compound named hexamminecobalt(III) sulphate?
A) [Co(NH3)6][SO4]
B) [Co(NH3)7][SO4]
C) [Co(NH3)6]2[SO3]3
D) [Co(NH3)6][SO4]2
E) [Co(NH3)6]2[SO4]3
A) [Co(NH3)6][SO4]
B) [Co(NH3)7][SO4]
C) [Co(NH3)6]2[SO3]3
D) [Co(NH3)6][SO4]2
E) [Co(NH3)6]2[SO4]3

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
11
What is the oxidation state of rhodium in pentaamminebromorhodium bromide, [Rh(NH3)5Br]Br2?
A) 0
B) +1
C) +2
D) +3
E) -2
A) 0
B) +1
C) +2
D) +3
E) -2

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
12
The complex Fe(C2O4)33- has one unpaired electron. What is the electron configuration of this complex?(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) t2g4eg1
B) t2g5eg1
C) t2g5
D) t2g4eg2
E) t2g3eg2
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) t2g4eg1
B) t2g5eg1
C) t2g5
D) t2g4eg2
E) t2g3eg2

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following has the most unpaired electrons?(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) [NiCl4]2-
B) [IrCl6]3-
C) [Cr(CN)6]3-
D) [Fe(NH3)6]2+
E) [Co(NH3)6]3+
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) [NiCl4]2-
B) [IrCl6]3-
C) [Cr(CN)6]3-
D) [Fe(NH3)6]2+
E) [Co(NH3)6]3+

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
14
You have two samples, one contains [Cr(CN)6]3- and the second [CrF6]3-. One solution is yellow, the second is green and they were found to have absorbance maxima at 650 and 380 nm. Unfortunately, you forgot to annotate which sample was which. Based on your knowledge which of the below show the correct assignments?(If needed, use the following equation:Spectrochemical Series I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) [Cr(CN)6]3- : yellow, 650 nm; [CrF6]3- : green, 380 nm
B) [Cr(CN)6]3- : yellow, 380 nm; [CrF6]3- : green, 650 nm
C) [Cr(CN)6]3- : green, 650 nm; [CrF6]3- :yellow, 380 nm
D) [Cr(CN)6]3- : green, 380 nm; [CrF6]3- : yellow, 650 nm
A) [Cr(CN)6]3- : yellow, 650 nm; [CrF6]3- : green, 380 nm
B) [Cr(CN)6]3- : yellow, 380 nm; [CrF6]3- : green, 650 nm
C) [Cr(CN)6]3- : green, 650 nm; [CrF6]3- :yellow, 380 nm
D) [Cr(CN)6]3- : green, 380 nm; [CrF6]3- : yellow, 650 nm

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
15
[Co(NH3)5NO2]Cl2 and [Co(NH3)5ONO]Cl2 are examples of what type of isomer?(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) coordination
B) optical
C) geometric
D) linkage
E) ionization
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) coordination
B) optical
C) geometric
D) linkage
E) ionization

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
16
The complexes Co(NH3)63+ and Mo(CO)6 are isoelectronic and diamagnetic. The first complex is orange and the second complex is white. What can you deduce about the value of in both of these complexes?(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) is larger in Mo(CO)6 than in Co(NH3)63+
B) is 0 in Mo(CO)6
C) is smaller in Mo(CO)6 than in Co(NH3)63+
D) is the same in the two complexes
E) is 0 in Co(NH3)63+
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) is larger in Mo(CO)6 than in Co(NH3)63+
B) is 0 in Mo(CO)6
C) is smaller in Mo(CO)6 than in Co(NH3)63+
D) is the same in the two complexes
E) is 0 in Co(NH3)63+

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
17
What differences might you expect between the two complexes,
Fe(CN)64- and FeF64-?(If needed, use the following equation:Spectrochemical Series I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) Fe(CN)64- will be pale yellow and paramagnetic and FeF64- will be coloured and diamagnetic.
B) Fe(CN)64- will be pale yellow and diamagnetic and FeF64- will be coloured and paramagnetic.
C) Fe(CN)64- will be coloured and paramagnetic and FeF64- will be pale yellow and diamagnetic.
D) Fe(CN)64- will be coloured and diamagnetic and FeF64- will be pale yellow and paramagnetic.
E) Fe(CN)64- will be coloured and paramagnetic and FeF64- will be coloured and paramagnetic.
Fe(CN)64- and FeF64-?(If needed, use the following equation:Spectrochemical Series I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) Fe(CN)64- will be pale yellow and paramagnetic and FeF64- will be coloured and diamagnetic.
B) Fe(CN)64- will be pale yellow and diamagnetic and FeF64- will be coloured and paramagnetic.
C) Fe(CN)64- will be coloured and paramagnetic and FeF64- will be pale yellow and diamagnetic.
D) Fe(CN)64- will be coloured and diamagnetic and FeF64- will be pale yellow and paramagnetic.
E) Fe(CN)64- will be coloured and paramagnetic and FeF64- will be coloured and paramagnetic.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
18
Aqueous copper(I) chloride is nearly colourless whereas aqueous copper(II) chloride is blue in colour. This difference is because(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) the crystal field splitting in copper(I) is much larger than that in copper(II).
B) the crystal field splitting in copper(I) is much smaller than that in copper(II).
C) the electron configuration of copper(II) has all subshells filled.
D) the electron configuration of copper(I) has all subshells filled.
E) copper(I) chloride doesn't dissolve in water.
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) the crystal field splitting in copper(I) is much larger than that in copper(II).
B) the crystal field splitting in copper(I) is much smaller than that in copper(II).
C) the electron configuration of copper(II) has all subshells filled.
D) the electron configuration of copper(I) has all subshells filled.
E) copper(I) chloride doesn't dissolve in water.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following five complexes are paramagnetic with 2 unpaired electrons? 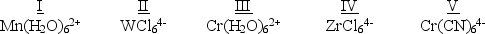 (If needed, use the following equation:Spectrochemical Series
(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) I and II
B) II, IV, and V
C) III
D) IV and V
E) I and III
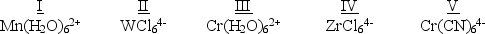 (If needed, use the following equation:Spectrochemical Series
(If needed, use the following equation:Spectrochemical Series I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) I and II
B) II, IV, and V
C) III
D) IV and V
E) I and III

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
20
How many chloride ions, Cl-, would you expect to find in a platinum complex described as PtCl4 . 5NH3?(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) 1
B) 2
C) 3
D) 4
E) 0
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
21
Arrange the following complexes in order of increasing orbital splitting enegy: [Co(NH3)6]3+, [Co(NH3)4]2+, [Co(NH3)6]2+.(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) [Co(NH3)6]3+, [Co(NH3)4]2+, [Co(NH3)6]2+
B) [Co(NH3)6]3+, [Co(NH3)6]2+, [Co(NH3)4]2+
C) [Co(NH3)4]2+, [Co(NH3)6]2+,[Co(NH3)6]3+
D) [Co(NH3)6]2+, [Co(NH3)4]2+,[Co(NH3)6]3+
E) [Co(NH3)4]2+, [Co(NH3)6]3+,[Co(NH3)6]2+
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) [Co(NH3)6]3+, [Co(NH3)4]2+, [Co(NH3)6]2+
B) [Co(NH3)6]3+, [Co(NH3)6]2+, [Co(NH3)4]2+
C) [Co(NH3)4]2+, [Co(NH3)6]2+,[Co(NH3)6]3+
D) [Co(NH3)6]2+, [Co(NH3)4]2+,[Co(NH3)6]3+
E) [Co(NH3)4]2+, [Co(NH3)6]3+,[Co(NH3)6]2+

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
22
In which of the following complexes would you expect the pairing energy to be larger than the orbital splitting energy?(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) FeCl6-4
B) Fe(CN)6-4
C) Fe(CN)6-3
D) W(CO)6
E) Fe(CO)6
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
A) FeCl6-4
B) Fe(CN)6-4
C) Fe(CN)6-3
D) W(CO)6
E) Fe(CO)6

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
23
The three main functions of metalloproteins are
A) transport and storage agents, catalysts to biochemical reactions, and redox agents.
B) transport and storage agents, enzymes, and electrons sinks.
C) transport and storage agents, inhibitors to biochemical reactions, and electron sinks.
D) inhibitors to biochemical reactions, redox agents, and agents to accelerate chemical reactions.
E) inhibitors to chemical reactions, electron sources, and oxidizing agents.
A) transport and storage agents, catalysts to biochemical reactions, and redox agents.
B) transport and storage agents, enzymes, and electrons sinks.
C) transport and storage agents, inhibitors to biochemical reactions, and electron sinks.
D) inhibitors to biochemical reactions, redox agents, and agents to accelerate chemical reactions.
E) inhibitors to chemical reactions, electron sources, and oxidizing agents.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
24
What is the oxidation state of iron in deoxyhaemoglobin and oxyhaemoglobin?
A) +2, +2
B) +3, +3
C) +2, +3
D) +3, +2
A) +2, +2
B) +3, +3
C) +2, +3
D) +3, +2

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
25
Deoxyhaemoglobin is bluish in colour, oxyhaemoglobin is bright red. This indicates that the crystal field splitting is
A) greater in deoxyhaemoglobin since blue light is higher energy than red light.
B) greater in oxyhaemoglobin since red light is higher in energy than blue light.
C) greater in deoxyhaemoglobin since high energy blue light is absorbed.
D) greater in oxyhaemoglobin since high energy blue light is absorbed.
E) greater in deoxyhaemoglobin since low energy red light is absorbed.
A) greater in deoxyhaemoglobin since blue light is higher energy than red light.
B) greater in oxyhaemoglobin since red light is higher in energy than blue light.
C) greater in deoxyhaemoglobin since high energy blue light is absorbed.
D) greater in oxyhaemoglobin since high energy blue light is absorbed.
E) greater in deoxyhaemoglobin since low energy red light is absorbed.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
26
What are reasons that metals are used in metalloproteins?
A) They help position the reactant molecules to facilitate the reaction.
B) They inhibit electron transfer.
C) They enhance the structural instability of the enzyme.
D) They bind irreversibly to ligands.
E) They contain many thousands of atoms.
A) They help position the reactant molecules to facilitate the reaction.
B) They inhibit electron transfer.
C) They enhance the structural instability of the enzyme.
D) They bind irreversibly to ligands.
E) They contain many thousands of atoms.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
27
What is at the active site for superoxide dismutase?
A) Cu+2
B) Zn+2, Cu+2
C) Fe+3 cytochrome
D) FeS cubane
E) Fe hemoglobin
A) Cu+2
B) Zn+2, Cu+2
C) Fe+3 cytochrome
D) FeS cubane
E) Fe hemoglobin

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the techniques in production of metals takes advantage of different redox properties?
A) roasting
B) leaching
C) refining
D) conversion
E) flotation
A) roasting
B) leaching
C) refining
D) conversion
E) flotation

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the techniques in production of metals takes advantage of different solubilities?
A) roasting
B) leaching
C) flotation
D) conversion
E) smelting
A) roasting
B) leaching
C) flotation
D) conversion
E) smelting

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
30
What is the layer above molten iron in a blast furnace called?
A) pig iron
B) water
C) impure oxides
D) slag
E) calcium carbonate
A) pig iron
B) water
C) impure oxides
D) slag
E) calcium carbonate

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
31
What process must be applied to ilmenite ore (FeTiO3) before it can be converted to titanium metal?
A) reaction with CO
B) reaction with CaO
C) reaction with HCl
D) reaction with Cl2 and C
E) reaction with HNO3
A) reaction with CO
B) reaction with CaO
C) reaction with HCl
D) reaction with Cl2 and C
E) reaction with HNO3

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following groups has primary use as catalysts?
A) group 4 metals (titanium family)
B) group 6 metals
C) the platinum metals
D) the group 11 metals
E) group 12 metals
A) group 4 metals (titanium family)
B) group 6 metals
C) the platinum metals
D) the group 11 metals
E) group 12 metals

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the metals is most likely to be found in elemental form in nature?
A) group 4 metals (titanium family)
B) group 6 metals
C) the platinum metals
D) the group 11 metals
E) group 12 metals
A) group 4 metals (titanium family)
B) group 6 metals
C) the platinum metals
D) the group 11 metals
E) group 12 metals

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
34
Which metal is primarily used in the photographic industry?
A) silver
B) copper
C) iron
D) silicon
E) cobalt
A) silver
B) copper
C) iron
D) silicon
E) cobalt

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
35
The metals of which group are most often used in batteries?
A) group 4 metals (titanium family)
B) group 6 metals
C) platinum
D) group 11 metals
E) group 12 metals
A) group 4 metals (titanium family)
B) group 6 metals
C) platinum
D) group 11 metals
E) group 12 metals

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
36
What is the material just one reaction away from chromium metal?
A) Na2CO3
B) FeCrO4
C) Cr2O3
D) Na2Cr2O7
E) Cr3+
A) Na2CO3
B) FeCrO4
C) Cr2O3
D) Na2Cr2O7
E) Cr3+

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
37
Titanium is used in many specialized engineering applications. Which of the following is characteristic of titanium?
A) high strength to weight ratio
B) ease of oxidation
C) high density
D) ease of fabrication
E) availability
A) high strength to weight ratio
B) ease of oxidation
C) high density
D) ease of fabrication
E) availability

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
38
Which metals were discovered first?
A) group 4
B) group 6
C) group 9
D) group 10
E) group 11
A) group 4
B) group 6
C) group 9
D) group 10
E) group 11

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck


