Deck 18: Macromolecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 18: Macromolecules
1
Describe functional groups and linkage groups in polymers.
Polymerization requires monomers that contain reactive functional groups. A condensation reaction produces a linkage group and eliminates a small molecule.
2
Describe polymers made by free radical polymerization.
Free radical polymerization includes initiation, propagation, and termination steps. Polymerizing mixtures of two different monomers to give copolymers increases the versatility of rubber materials. Cross-linking strengthens rubber material by creating chemical links between long-chain molecules.
3
Describe polymers made by condensation polymerization.
Each monomer in a condensation polymerization has two functional groups. Polyamides include nylons, Kevlar®, and proteins. Polyesters include poly(ethylene terephthalate), Dacron, and Mylar.
4
Recognize and describe some properties of plastics, fibres, and elastomers.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
Recognize and draw structures of monosaccharides and polysaccharides.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
Draw primary and secondary structures of DNA and RNA.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
Explain primary, secondary, and tertiary structures of proteins.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
How do bond electrons differ from bond electrons in C == C bonds?
A) bond electrons are located along the bond axis, and bond electrons are located above and below the plane of the bond axis.
B) bond electrons are more tightly bound that bond electrons thus explaining why double bonds are stronger than single bonds.
C) bond electrons dominate the reactivity patterns of ethylene as they are more readily accessible.
D) bond electrons are located along the bond axis, and bond electrons are located above and below the plane of the bond axis.
E) Both kinds of electrons are essentially equivalent.
A) bond electrons are located along the bond axis, and bond electrons are located above and below the plane of the bond axis.
B) bond electrons are more tightly bound that bond electrons thus explaining why double bonds are stronger than single bonds.
C) bond electrons dominate the reactivity patterns of ethylene as they are more readily accessible.
D) bond electrons are located along the bond axis, and bond electrons are located above and below the plane of the bond axis.
E) Both kinds of electrons are essentially equivalent.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following are important polymer linkage groups?
I. ester
II. Carboxyl
III. Amide
IV. Amine
V. ether
A) I, IV and V
B) I, III and V
C) I, II,and V
D) I, II and III
E) III and IV
I. ester
II. Carboxyl
III. Amide
IV. Amine
V. ether
A) I, IV and V
B) I, III and V
C) I, II,and V
D) I, II and III
E) III and IV

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
What is the chemical formula for the following compound? 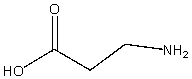
A) C3H7O2N
B) C5H9O2N
C) C3H9ON2
D) C4H8O2N
E) C3H4ON

A) C3H7O2N
B) C5H9O2N
C) C3H9ON2
D) C4H8O2N
E) C3H4ON

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
What functional groups are present in the ball and stick model shown below? 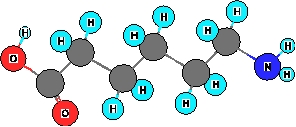
I. amine
II. Alcohol
III. carboxylic acid
IV. Ketone
V. thiol
A) I and IV
B) I and II
C) I and V
D) I and III
E) III only

I. amine
II. Alcohol
III. carboxylic acid
IV. Ketone
V. thiol
A) I and IV
B) I and II
C) I and V
D) I and III
E) III only

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
What is the linkage group and chemical formula for the following compound? 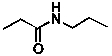
A) amine, C6H12NO
B) amine, C6H13NO
C) carbonyl, C6H13NO
D) amide, C6H12NO
E) amide, C6H13NO

A) amine, C6H12NO
B) amine, C6H13NO
C) carbonyl, C6H13NO
D) amide, C6H12NO
E) amide, C6H13NO

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following will result in termination of polymer growth?
A) reaction of the radical chain with another alkene
B) reaction of the radical chain with a carbonyl
C) reaction of the radical chain with another radical chain
D) reaction of an two alkenes
E) absorption of light
A) reaction of the radical chain with another alkene
B) reaction of the radical chain with a carbonyl
C) reaction of the radical chain with another radical chain
D) reaction of an two alkenes
E) absorption of light

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following would be a suitable initiator molecule for polymerization of ethylene?
A) HO-
B) HO .
C) H2O
D) H3O+
A) HO-
B) HO .
C) H2O
D) H3O+

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
What is typically used as an initiation step for radical polymerization?
A) two alkenes reacting together
B) a peroxide bond being cleaved
C) two radicals reacting together
D) a radical reacting with an alkene
E) reaction of a radical with cleaved peroxide
A) two alkenes reacting together
B) a peroxide bond being cleaved
C) two radicals reacting together
D) a radical reacting with an alkene
E) reaction of a radical with cleaved peroxide

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
What is a typically propagation step for radical polymerization?
A) two alkenes reacting together
B) a peroxide bond being cleaved
C) two radicals reacting together
D) a radical reacting with an alkene
E) reaction of a radical with cleaved peroxide
A) two alkenes reacting together
B) a peroxide bond being cleaved
C) two radicals reacting together
D) a radical reacting with an alkene
E) reaction of a radical with cleaved peroxide

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
What is a typically termination step for radical polymerization?
A) two alkenes reacting together
B) a peroxide bond being cleaved
C) two radicals reacting together
D) a radical reacting with an alkene
E) reaction of an alcohol with an carboxylic acid
A) two alkenes reacting together
B) a peroxide bond being cleaved
C) two radicals reacting together
D) a radical reacting with an alkene
E) reaction of an alcohol with an carboxylic acid

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
What could a side group such as the one shown below be used for on a polymer? 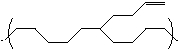
A) used to make an elastomer
B) used to cross-link polymer chains
C) used to terminate
D) used in high density polymers
E) used to make a brittle polymer

A) used to make an elastomer
B) used to cross-link polymer chains
C) used to terminate
D) used in high density polymers
E) used to make a brittle polymer

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following are likely to participate in a condensation polymerization reaction?
A) ethylene glycol, HOCH2CH2OH
B) tetrafluorethylene, C2F4
C) propylene, C3H6
D) styrene, CH2CHC6H6
E) peroxide, HOOH
A) ethylene glycol, HOCH2CH2OH
B) tetrafluorethylene, C2F4
C) propylene, C3H6
D) styrene, CH2CHC6H6
E) peroxide, HOOH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
What bonds CANNOT be easily broken in the process of polymerization?
A) C=C
B) C-C
C) C-OH
D) N-H
E) O-H
A) C=C
B) C-C
C) C-OH
D) N-H
E) O-H

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
Thermoplastic materials
A) retain their structural integrity.
B) are highly cross-linked.
C) decompose irreversible when heated.
D) melt or deform on heating.
E) are always soft and semi-rigid.
A) retain their structural integrity.
B) are highly cross-linked.
C) decompose irreversible when heated.
D) melt or deform on heating.
E) are always soft and semi-rigid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
High density polyethylene
A) forms under conditions that produce branched chain CH2 groups.
B) forms from linear molecules which maximize dispersion forces between aligned polymer molecules.
C) forms from branched chained polymers which maximizes the dispersion forces as molecules fit together like puzzle pieces.
D) is an amorphous polymer that melts at relatively low temperature.
E) is flexible and ideal for making squeeze bottles.
A) forms under conditions that produce branched chain CH2 groups.
B) forms from linear molecules which maximize dispersion forces between aligned polymer molecules.
C) forms from branched chained polymers which maximizes the dispersion forces as molecules fit together like puzzle pieces.
D) is an amorphous polymer that melts at relatively low temperature.
E) is flexible and ideal for making squeeze bottles.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
Increasing the degree of cross-linking in a polymer
A) increases flexibility and increases strength.
B) decreases flexibility and decreases strength.
C) increases flexibility and decreases strength.
D) decreases flexibility and increases strength.
A) increases flexibility and increases strength.
B) decreases flexibility and decreases strength.
C) increases flexibility and decreases strength.
D) decreases flexibility and increases strength.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
How would you make a polymer that is strong and rigid for a container?
A) have branches on the polymer
B) cross-linking
C) add a metal catalyst
D) add an ingredient to hydrogen bond with the polymer
E) have more double bonds
A) have branches on the polymer
B) cross-linking
C) add a metal catalyst
D) add an ingredient to hydrogen bond with the polymer
E) have more double bonds

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
How would you make a polymer that is flexible and low in density?
A) have branches on the polymer
B) cross-linking
C) use a polymer with no branches
D) co-polymerizing
E) add a metal catalyst
A) have branches on the polymer
B) cross-linking
C) use a polymer with no branches
D) co-polymerizing
E) add a metal catalyst

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
Cross-linking polymers results in
A) lower viscosity.
B) lower tensile strength.
C) greater density.
D) greater rigidity.
E) greater flexibility.
A) lower viscosity.
B) lower tensile strength.
C) greater density.
D) greater rigidity.
E) greater flexibility.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
What type of polymer would be best suited for a case that will contain heat-emitting equipment?
A) thermoplastic
B) thermoset
C) elastomer
D) fibre
E) lightly cross-linked
A) thermoplastic
B) thermoset
C) elastomer
D) fibre
E) lightly cross-linked

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
What type of polymer would be best suited for an automobile bumper?
A) thermoplastic
B) thermoset
C) elastomer
D) fibre
E) lightly cross-linked
A) thermoplastic
B) thermoset
C) elastomer
D) fibre
E) lightly cross-linked

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
Nylon rope, a polyamide fibre product, has a tendency to stretch when it becomes wet (during a rainstorm, for example). Why?
A) The water molecules act like plasticizers.
B) The water leads to cooling of the rope.
C) The nylon polymer molecules degrade in the presence of water.
D) The water molecules disrupt the intramolecular H bonds.
E) Cross-linkages are dissolved in water.
A) The water molecules act like plasticizers.
B) The water leads to cooling of the rope.
C) The nylon polymer molecules degrade in the presence of water.
D) The water molecules disrupt the intramolecular H bonds.
E) Cross-linkages are dissolved in water.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
Bungee jumpers use cords which
A) have a large degree of all cis configurations of double bonds.
B) have a large degree of all trans configurations of double bonds.
C) are made of a thermoset polymer.
D) are made of a highly cross-linked elastomer.
E) are made of Kevlar.
A) have a large degree of all cis configurations of double bonds.
B) have a large degree of all trans configurations of double bonds.
C) are made of a thermoset polymer.
D) are made of a highly cross-linked elastomer.
E) are made of Kevlar.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the polymer types listed below is most suitable for recycling?
A) thermoset
B) elastomer
C) urea-formaldehyde polymers
D) thermoplastics
E) vulcanized rubbers
A) thermoset
B) elastomer
C) urea-formaldehyde polymers
D) thermoplastics
E) vulcanized rubbers

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
A glycosidic bond between two monosaccharides results in the formation of what two molecules?
A) water and disaccharide
B) disaccharide and starch
C) polysaccharide and water
D) starch and water
E) cellulose and glycogen
A) water and disaccharide
B) disaccharide and starch
C) polysaccharide and water
D) starch and water
E) cellulose and glycogen

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
A glycosidic bond is made of what type of functional group?
A) amide
B) ester
C) ether
D) ketone
E) polyester
A) amide
B) ester
C) ether
D) ketone
E) polyester

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
Olestra is a new oil substitute in the market where a hexose has fatty acids attached to the sugar via glycosidic bonds. What is the maximum number of glycosidic bonds possible in a hexose?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Starch is
A) a disaccharide formed from -glucose.
B) a disaccharide formed from -glucose.
C) a polysaccharide formed from -glucose.
D) a polysaccharide formed from both -glucose and -glucose.
E) a polysaccharide formed from the disaccharide amylase.
A) a disaccharide formed from -glucose.
B) a disaccharide formed from -glucose.
C) a polysaccharide formed from -glucose.
D) a polysaccharide formed from both -glucose and -glucose.
E) a polysaccharide formed from the disaccharide amylase.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
Most animals can digest starch but NOT cellulose. What is the major difference between starch and cellulose?
A) type of sugar unit used to make the polymer
B) length of the polymer
C) type of glycosidic linkage
D) tertiary structure
E) molecular weight of the polymers
A) type of sugar unit used to make the polymer
B) length of the polymer
C) type of glycosidic linkage
D) tertiary structure
E) molecular weight of the polymers

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
What two functional groups are used to combine monosaccharides into polymers?
A) two alcohol groups
B) one alcohol and one carboxylic acid group
C) one alcohol and one amine group
D) one amine and one carboxylic acid group
E) two amine groups
A) two alcohol groups
B) one alcohol and one carboxylic acid group
C) one alcohol and one amine group
D) one amine and one carboxylic acid group
E) two amine groups

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
What intermolecular force keeps cellulose from moving past each other?
A) dispersion forces
B) hydrogen bonding
C) dipole interactions
D) ionic interactions
E) multiple cross-linking
A) dispersion forces
B) hydrogen bonding
C) dipole interactions
D) ionic interactions
E) multiple cross-linking

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
The mass, in nanograms, for a single DNA molecule (molar mass ca. 109 g/mol) is
A) 1.7 x 106 ng.
B) 1.7 x 10-15 ng.
C) 1.7 x 10-6 ng.
D) 6.0 x 1014 ng.
E) 6.0 x 105 ng.
A) 1.7 x 106 ng.
B) 1.7 x 10-15 ng.
C) 1.7 x 10-6 ng.
D) 6.0 x 1014 ng.
E) 6.0 x 105 ng.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
What type of bonding holds the double stranded DNA together?
A) ionic
B) dipole interactions
C) hydrogen bonding
D) covalent bonding
E) dispersion forces
A) ionic
B) dipole interactions
C) hydrogen bonding
D) covalent bonding
E) dispersion forces

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
Nucleic acids include which of the following?
I. nitrogen containing organic base
II. glycosidic linkage
III. pentose sugar
IV. carbonate linkage
A) I, II, III and IV
B) I and II
C) I and III
D) II and III
E) IV and I
I. nitrogen containing organic base
II. glycosidic linkage
III. pentose sugar
IV. carbonate linkage
A) I, II, III and IV
B) I and II
C) I and III
D) II and III
E) IV and I

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
What type of bond is formed when adding DNA units onto a DNA strand?
A) glycosidic linkage
B) amide linkage
C) hydrogen bonds
D) phosphate linkage
E) ester linkage
A) glycosidic linkage
B) amide linkage
C) hydrogen bonds
D) phosphate linkage
E) ester linkage

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the amino acids are hydrophobic? 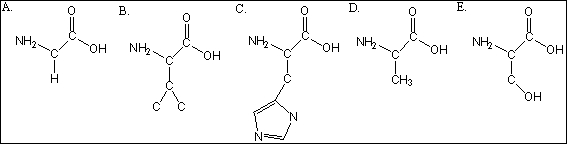
A) A
B) A, B
C) A, B, C
D) A, B, D
E) B, E

A) A
B) A, B
C) A, B, C
D) A, B, D
E) B, E

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
Primary, secondary and tertiary structure of proteins is determined by
A) sequence of amino acids, hydrogen bonds, interaction of side chains with water.
B) sequence of amino acids, peptide linkages, hydrogen bonds.
C) order of the dipeptides, sequence of amino acids, hydrogen bonds.
D) number of dipeptides, hydrogen bonds, interactions of side chains with water.
E) sequence of amino acids, hydrogen bonds, double helix shape.
A) sequence of amino acids, hydrogen bonds, interaction of side chains with water.
B) sequence of amino acids, peptide linkages, hydrogen bonds.
C) order of the dipeptides, sequence of amino acids, hydrogen bonds.
D) number of dipeptides, hydrogen bonds, interactions of side chains with water.
E) sequence of amino acids, hydrogen bonds, double helix shape.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
How many products containing two amino acids can be formed from the following three amino acids? 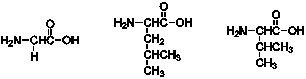
A) 3
B) 4
C) 5
D) 6
E) 7

A) 3
B) 4
C) 5
D) 6
E) 7

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
What chemical bonding would characterize how proteins form secondary structures like beta sheets?
A) covalent bonding
B) thiol bonds (disulfide bridge)
C) ionic bonds
D) hydrogen bonding
E) dispersion forces
A) covalent bonding
B) thiol bonds (disulfide bridge)
C) ionic bonds
D) hydrogen bonding
E) dispersion forces

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
Proteins CANNOT be which of the following?
A) metal storage centres
B) oxidation-reduction centres
C) n2 transporters
D) reaction catalysts
E) sources of energy
A) metal storage centres
B) oxidation-reduction centres
C) n2 transporters
D) reaction catalysts
E) sources of energy

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
What chemical bonding would characterize how proteins are "locked" into tertiary structures?
A) dipole bonding
B) thiol bonds (disulfide bridge)
C) amide bonds
D) hydrogen bonding
E) dispersion forces
A) dipole bonding
B) thiol bonds (disulfide bridge)
C) amide bonds
D) hydrogen bonding
E) dispersion forces

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
Use the following amino acids for questions
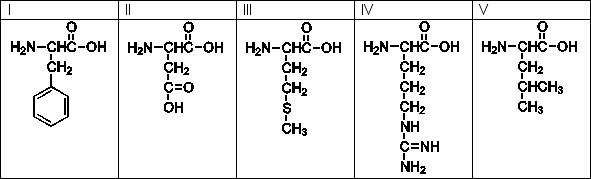
-Which of the amino acids has a hydrophobic side chain?
A) II, IV
B) I, II, III
C) III, IV, V
D) I, III, IV
E) I, III, V

-Which of the amino acids has a hydrophobic side chain?
A) II, IV
B) I, II, III
C) III, IV, V
D) I, III, IV
E) I, III, V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
Use the following amino acids for questions

-Which of the above is the line structure for tyrosine?
A) I
B) II
C) III
D) IV

-Which of the above is the line structure for tyrosine?
A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
Use the following amino acids for questions

-Which of the above is the line structure for methionine?
A) I
B) II
C) III
D) IV
E) V

-Which of the above is the line structure for methionine?
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
Fill in the missing entries: 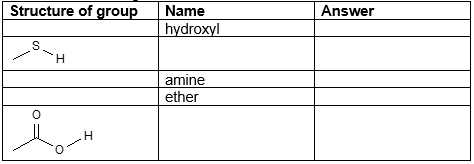


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
Draw a representative molecule containing three carbons for each of the following. 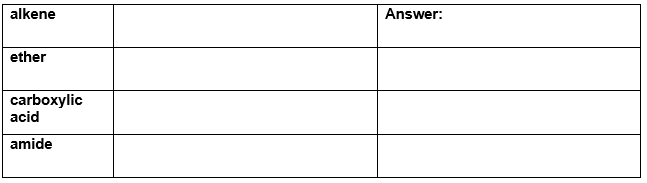


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
Draw a representative molecule containing three carbons for each of the following. 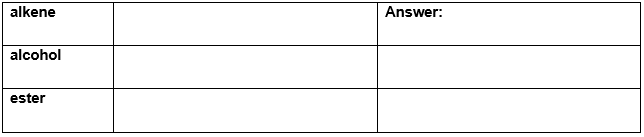


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the product of the condensation reaction between dimethylamine (HN(CH3)2) and formic acid (H-C=O)-OH) 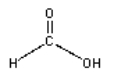
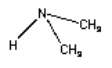 H-(C= O)-OH H-N-(CH3)2
H-(C= O)-OH H-N-(CH3)2

 H-(C= O)-OH H-N-(CH3)2
H-(C= O)-OH H-N-(CH3)2
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
Draw the product of the condensation reaction of 1-propanol with itself. CH3CH2CH2OH.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
Draw the structures of two molecules that would form the molecule shown below in a condensation reaction. 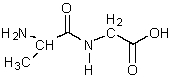


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
What is the expected product of the reaction between methanol (CH3OH) and phosphoric acid (H3PO4)?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
What is the line structure for the compound containing an amide-linking group? 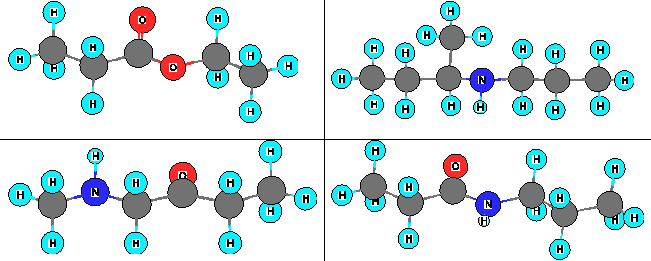


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
From what monomer is acrylonitrile made from if the polymer looks like: 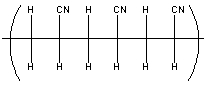


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
Draw a line structure for a segment of the polymer polyvinyl chloride containing three monomer units (H2C=CHCl).

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
The compound 1,5-hexadiene (below) has two double bonds that can participate in adding to polymers. 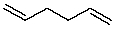 1,5-Hexadiene: Draw a segment of a co-polymer of 1,5-hexadiene and ethylene in which only one double bond of 1,5-hexadiene is involved in the polymerization process. The ratio of ethylene to 1,5-hexadiene is 4:1.
1,5-Hexadiene: Draw a segment of a co-polymer of 1,5-hexadiene and ethylene in which only one double bond of 1,5-hexadiene is involved in the polymerization process. The ratio of ethylene to 1,5-hexadiene is 4:1.
 1,5-Hexadiene: Draw a segment of a co-polymer of 1,5-hexadiene and ethylene in which only one double bond of 1,5-hexadiene is involved in the polymerization process. The ratio of ethylene to 1,5-hexadiene is 4:1.
1,5-Hexadiene: Draw a segment of a co-polymer of 1,5-hexadiene and ethylene in which only one double bond of 1,5-hexadiene is involved in the polymerization process. The ratio of ethylene to 1,5-hexadiene is 4:1.
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
Draw the structure of the polymer made from cyclopentene, whose structure is shown below. 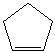


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
How would a scientist make a co-polymer via radical polymerization out of two monomer units with a ratio of 3 to 1? Would the scientist be able to control the order of the addition of monomer units? That is, could the scientist guarantee that the order would be A-A-A-B-A-A-A-B-A-A-A-B- and so on? If so how?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
Indentify the linkage group and the molecule eliminated when adipic acid, (CH2)4(COOH)2 and hexamethylenediamine, H2N(CH2)6NH2, polymerize via a condensation reaction to form Nylon 6,6.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
The polymer Lexan, shown below, is a condensation polymer formed with the elimination of an HCl molecule. Identify the monomers. 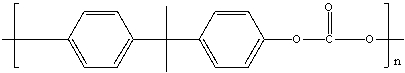


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
Dacron is a polyester with the following structure. 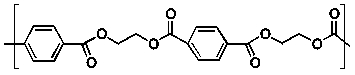 What is the structure of the monomer containing a phenyl group used to make this polymer?
What is the structure of the monomer containing a phenyl group used to make this polymer?
 What is the structure of the monomer containing a phenyl group used to make this polymer?
What is the structure of the monomer containing a phenyl group used to make this polymer?
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
A polymer is being made and marketed by Cargill. This polymer is made from lactic acid, a by-product of ethanol production from corn. Draw the product of three lactic acids combined via condensation reactions. 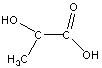


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
Sketch the polymer formed from the condensation of the molecules below. Include at least two units of each in your sketch. 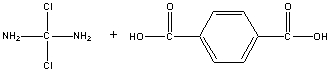


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
One way to synthesize polyamides is from acid chlorides and amines with the loss of HCl. Draw the structure of the combination of two units of each of the following monomers: 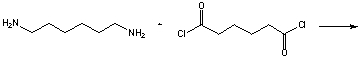


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
Sketch a polymer made from the polymerization of three units of 1,3 dipropyl alcohol (HOCH2CH2CH2OH ) via condensation reactions.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
Butadiene, CH2=CH-CH=CH2 (line drawing below), forms polymers that are used in elastomers. Write the structure of polybutadiene (at least three units included) and explain why this polymer would be more elastic than polyethylene. 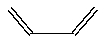


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
Complete the picture of ? -glucose where the information for carbons 1 and 5 have been left off. 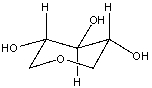


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
The structure shown below is -glucose. If -galactose differs from -glucose only in the orientation of groups on the carbon in the fourth position, and -glucose differs from -glucose only in the orientation of groups on the carbon adjacent to the ring oxygen, draw the structure for -galactose. -glucose 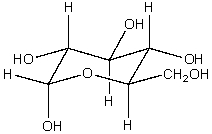


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
What is the complementary structure for the following DNA base sequence: TGGCTTAAT?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
If the primary structure of a segment of RNA is UUGCAUUGC, what sequence of DNA was this transcribed from?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
Draw the structure of the nucleotide formed from the base adenine, ribose and phosphate shown below: 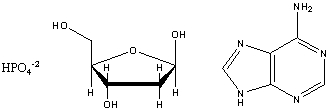


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
Draw the structure of the nucleotide formed from the base thymine, ribose and phosphate shown below: 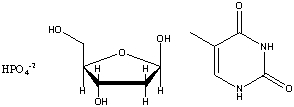


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
Draw the four nucleotide nucleic acid with primary structure TTGC. Use the shorthand notation.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
Use the following amino acid structures to answer questions
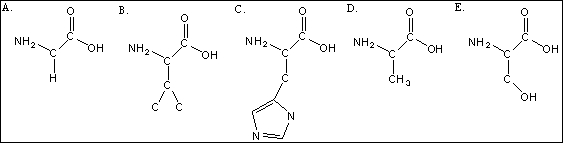
-Give the formula for amino acid shown above as B.

-Give the formula for amino acid shown above as B.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


