Exam 18: Macromolecules
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
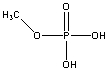
What is the expected product of the reaction between methanol (CH3OH) and phosphoric acid (H3PO4)?
Free
(Essay)
4.9/5  (43)
(43)
Correct Answer:
Recognize and draw structures of monosaccharides and polysaccharides.
Free
(Essay)
4.9/5  (34)
(34)
Correct Answer:
Monosaccharides have the formula (CH2O)n, where n is between 3 and 6. A C-O-C linkage between two sugar molecules is termed a glycosidic bond. Polysaccharides in biological organisms act as structural materials or as reservoirs for energy storage.
The mass, in nanograms, for a single DNA molecule (molar mass ca. 109 g/mol) is
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
C
Which of the following are important polymer linkage groups?
I. ester
II. Carboxyl
III. Amide
IV. Amine
V. ether
(Multiple Choice)
4.8/5  (30)
(30)
How would you make a polymer that is flexible and low in density?
(Multiple Choice)
4.7/5  (41)
(41)
How would you make a polymer that is strong and rigid for a container?
(Multiple Choice)
4.8/5  (34)
(34)
The compound 1,5-hexadiene (below) has two double bonds that can participate in adding to polymers. 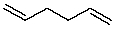 1,5-Hexadiene: Draw a segment of a co-polymer of 1,5-hexadiene and ethylene in which only one double bond of 1,5-hexadiene is involved in the polymerization process. The ratio of ethylene to 1,5-hexadiene is 4:1.
1,5-Hexadiene: Draw a segment of a co-polymer of 1,5-hexadiene and ethylene in which only one double bond of 1,5-hexadiene is involved in the polymerization process. The ratio of ethylene to 1,5-hexadiene is 4:1.
(Essay)
4.8/5  (32)
(32)
What could a side group such as the one shown below be used for on a polymer? 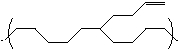
(Multiple Choice)
4.7/5  (44)
(44)
What type of polymer would be best suited for a case that will contain heat-emitting equipment?
(Multiple Choice)
4.9/5  (35)
(35)
What is the complementary structure for the following DNA base sequence: TGGCTTAAT?
(Short Answer)
4.9/5  (36)
(36)
Nucleic acids include which of the following?
I. nitrogen containing organic base
II. glycosidic linkage
III. pentose sugar
IV. carbonate linkage
(Multiple Choice)
4.8/5  (35)
(35)
Use the following amino acid structures to answer questions
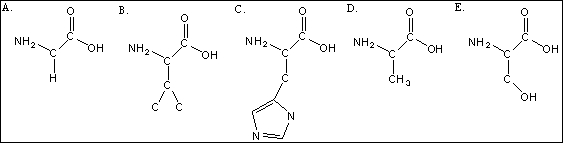 -Sketch the sequence of the amino acid sequence, B-D-B-E, made via condensation reactions.
-Sketch the sequence of the amino acid sequence, B-D-B-E, made via condensation reactions.
(Essay)
4.7/5  (37)
(37)
Most animals can digest starch but NOT cellulose. What is the major difference between starch and cellulose?
(Multiple Choice)
4.8/5  (30)
(30)
Sketch a polymer made from the polymerization of three units of 1,3 dipropyl alcohol (HOCH2CH2CH2OH ) via condensation reactions.
(Essay)
4.9/5  (36)
(36)
Indentify the linkage group and the molecule eliminated when adipic acid, (CH2)4(COOH)2 and hexamethylenediamine, H2N(CH2)6NH2, polymerize via a condensation reaction to form Nylon 6,6.
(Short Answer)
4.9/5  (43)
(43)
The structure shown below is -glucose. If -galactose differs from -glucose only in the orientation of groups on the carbon in the fourth position, and -glucose differs from -glucose only in the orientation of groups on the carbon adjacent to the ring oxygen, draw the structure for -galactose. -glucose 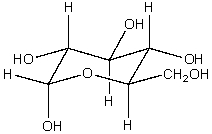
(Essay)
4.9/5  (47)
(47)
What is a typically propagation step for radical polymerization?
(Multiple Choice)
4.7/5  (32)
(32)
What type of bonding holds the double stranded DNA together?
(Multiple Choice)
4.8/5  (37)
(37)
Draw a line structure for a segment of the polymer polyvinyl chloride containing three monomer units (H2C=CHCl).
(Essay)
4.9/5  (33)
(33)
Showing 1 - 20 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)