Deck 16: Applications of Aqueous Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 16: Applications of Aqueous Equilibria
1
Calculate the pH of a buffered solution.
A buffer solution contains both a weak acid and its conjugate base, or a weak base and its conjugate acid, as major species in solution.
2
Explain how to prepare a buffered solution of known pH and capacity.
NOT ANSWER
3
Calculate an acid or a base concentration from titration data.
At the stoichiometric point, just enough hydroxide ions have been added to react with every acidic proton present in the acid solution before the titration was started.
4
Use the concepts of Ksp and the common-ion effect to calculate solution concentrations.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the concentrations of species involved in complex-ion formation.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
When 0.1 moles of HC2H3O2 and 0.1 moles of NaC2H3O2 are added to make 1 L of aqueous solution, the major species present are
A) Na+, H2O and HC2H3O2.
B) HC2H3O2 and C2H3O2-.
C) H2O, Na+ and C2H3O2-.
D) NaC2H3O2, HC2H3O2 and H2O.
E) Na+, H2O, HC2H3O2, C2H3O2-.
A) Na+, H2O and HC2H3O2.
B) HC2H3O2 and C2H3O2-.
C) H2O, Na+ and C2H3O2-.
D) NaC2H3O2, HC2H3O2 and H2O.
E) Na+, H2O, HC2H3O2, C2H3O2-.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
When 0.1 moles of HCl are added to 1 L of solution containing 0.12 moles of aqueous Na2CO3, the major species present are
A) Na+, Cl-, H2O, HCO3-.
B) Na+, Cl-, H2O, CO32-.
C) Na+, Cl-, H2O, HCO3-, CO32-.
D) HCl, Na+, CO32-.
E) Na+, Cl-, H2O, H2CO3, CO32.
A) Na+, Cl-, H2O, HCO3-.
B) Na+, Cl-, H2O, CO32-.
C) Na+, Cl-, H2O, HCO3-, CO32-.
D) HCl, Na+, CO32-.
E) Na+, Cl-, H2O, H2CO3, CO32.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
What are the major species present when 10 ml of 0.1 M NaOH are added to 40 ml of 0.0.05 M CH3COOH?
A) Na+, OH-, H2O and CH3COOH
B) CH3COOH and CH3COO-
C) H2O, Na+ and CH3COO-
D) NaOH, CH3COOH and H2O
E) Na+, H2O, CH3COOH, CH3COO-
A) Na+, OH-, H2O and CH3COOH
B) CH3COOH and CH3COO-
C) H2O, Na+ and CH3COO-
D) NaOH, CH3COOH and H2O
E) Na+, H2O, CH3COOH, CH3COO-

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following mixtures would make a buffer of pH = 4.74? (pKa of acetic acid is 4.74)
A) 100 mL of 0.10 M HCl and 100 mL of 0.10 M NaC2H3O2
B) 100 mL of 0.10 M HCl and 100 mL of 0.10 M HC2H3O2
C) 50 mL of 0.10 M HC2H3O2 and 100 mL of 0.10 M NaC2H3O2
D) 100 mL of 0.10 M NaOH and 100 mL of 0.10 M NaC2H3O2
E) 50 mL of 0.10 M NaOH and 100 mL of 0.10 M HC2H3O2
A) 100 mL of 0.10 M HCl and 100 mL of 0.10 M NaC2H3O2
B) 100 mL of 0.10 M HCl and 100 mL of 0.10 M HC2H3O2
C) 50 mL of 0.10 M HC2H3O2 and 100 mL of 0.10 M NaC2H3O2
D) 100 mL of 0.10 M NaOH and 100 mL of 0.10 M NaC2H3O2
E) 50 mL of 0.10 M NaOH and 100 mL of 0.10 M HC2H3O2

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following solutions is a buffer?
I. 50 mL of 0.1 M acetic acid mixed with 25 mL of 0.05 M NaOH
II. 100 mL of 0.1 M acetic acid mixed with 25 mL of 0.5 M NaOH
III. 50 mL of 0.1 M acetic acid mixed with 25 mL of 0.05 M sodium acetate
IV. 50 mL of 0.1 M acetic acid mixed with 25 mL of 0.2 M HCl
V. 50 mL of 0.1 M sodium acetate mixed with 25 mL of 0.05 M NaOH
A) I and III
B) III only
C) I, II and V
D) III, IV and V
E) II, IV and V
I. 50 mL of 0.1 M acetic acid mixed with 25 mL of 0.05 M NaOH
II. 100 mL of 0.1 M acetic acid mixed with 25 mL of 0.5 M NaOH
III. 50 mL of 0.1 M acetic acid mixed with 25 mL of 0.05 M sodium acetate
IV. 50 mL of 0.1 M acetic acid mixed with 25 mL of 0.2 M HCl
V. 50 mL of 0.1 M sodium acetate mixed with 25 mL of 0.05 M NaOH
A) I and III
B) III only
C) I, II and V
D) III, IV and V
E) II, IV and V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
A solution is made by the addition of 0.34 mole of Na2HPO4 and 0.65 mole of NaH2PO4 and sufficient water to give a total volume of 1.2 L. How many grams of NaOH would need to be added to increase the pH by 0.2 pH units?If needed, use the following equation: pH = pKa + log(A-/HA)
A) 0.34
B) 3.4
C) 4.4
D) 5.4
E) 6.5
A) 0.34
B) 3.4
C) 4.4
D) 5.4
E) 6.5

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
Two buffer solutions of the same pH are prepared. After adding 15 millimoles of acid to 250 mL of one solution, the pH changes by 0.02 pH unit. Adding 15 millimoles of acid to 250 mL of the second solution results in a 0.05 pH unit change. Which statement most accurately describes the differences between the two buffer solutions?
A) The first solution has a lower capacity than the second solution.
B) The first solution has a higher concentration of conjugate acid than conjugate base.
C) The second solution has a lower concentration of conjugate base that the first solution.
D) The second solution has a higher acid to conjugate base ratio than the first solution.
E) The first solution has a higher capacity than the second solution.
A) The first solution has a lower capacity than the second solution.
B) The first solution has a higher concentration of conjugate acid than conjugate base.
C) The second solution has a lower concentration of conjugate base that the first solution.
D) The second solution has a higher acid to conjugate base ratio than the first solution.
E) The first solution has a higher capacity than the second solution.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
The pKa's of the three acid-conjugate base pairs derived from phosphoric acid, H3PO4, are 2.12, 7.21 and 12.32. How many g of sodium hydroxide would have be added to 150 mL of 0.1 M H3PO4 to prepare a buffer of pH = 7.41?
A) 0. 21 g
B) 0.34 g
C) 0.56 g
D) 0.83 g
E) 0.91 g
A) 0. 21 g
B) 0.34 g
C) 0.56 g
D) 0.83 g
E) 0.91 g

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
A biochemist wishes to study the activity of an enzyme that produces one mole hydrogen ion for every mole of product produced. However, the enzyme is sensitive to changes in pH and will become inactive at pH less than 7.1. If the initial pH of the solution is 7.21 set with a phosphate buffer (the pKa's of the three acid-conjugate base pairs derived from phosphoric acid, H3PO4, are 2.12, 7.21 and 12.32) and the reaction is designed to produce 0.15 moles of product in a volume of 250 mL, what is the minimum concentration of the conjugate acid in the buffer solution?
A) 0.46 M
B) 0.53 M
C) 0.82 M
D) 1.12 M
E) 1.25 M
A) 0.46 M
B) 0.53 M
C) 0.82 M
D) 1.12 M
E) 1.25 M

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following will have the smallest pH change upon the addition of 0.001 mole strong acid?
A) 50 mL of a total concentration of 0.10 M pH = 5.2 acetate buffer
B) 100 mL of a total concentration of 0.050 M pH = 5.2 acetate buffer
C) 75 mL of a total concentration of 0.067 M pH = 5.2 acetate buffer
D) 250 mL of a total concentration of 0.020 M pH = 5.2 acetate buffer
E) 25 ml of total concentration of 0.20 M pH = 5.2 acetate buffer
A) 50 mL of a total concentration of 0.10 M pH = 5.2 acetate buffer
B) 100 mL of a total concentration of 0.050 M pH = 5.2 acetate buffer
C) 75 mL of a total concentration of 0.067 M pH = 5.2 acetate buffer
D) 250 mL of a total concentration of 0.020 M pH = 5.2 acetate buffer
E) 25 ml of total concentration of 0.20 M pH = 5.2 acetate buffer

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
In which of the following titrations will the pH be less than 7 at the equivalence point?
A) acetic acid titrated with sodium hydroxide
B) perchloric acid titrated with lithium hydroxide
C) sodium hydroxide titrated with hydrochloric acid
D) ammonium bromide titrated with perchloric acid
E) perchloric acid titrated with methyl amine
A) acetic acid titrated with sodium hydroxide
B) perchloric acid titrated with lithium hydroxide
C) sodium hydroxide titrated with hydrochloric acid
D) ammonium bromide titrated with perchloric acid
E) perchloric acid titrated with methyl amine

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
In which of the following titrations will the pH be greater than 7 at the equivalence point?
A) perchloric acid titrated with lithium hydroxide
B) acetic acid titrated with sodium hydroxide
C) sodium hydroxide titrated with hydrochloric acid
D) ammonium bromide titrated with perchloric acid
E) perchloric acid titrated with methyl amine
A) perchloric acid titrated with lithium hydroxide
B) acetic acid titrated with sodium hydroxide
C) sodium hydroxide titrated with hydrochloric acid
D) ammonium bromide titrated with perchloric acid
E) perchloric acid titrated with methyl amine

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
The equivalence point for titration of 50 ml of 0.1 M formic acid, HCO2H (a weak acid) requires what volume of 0.2 M sodium hydroxide?
A) 50 ml
B) 25 ml
C) less than 25 ml as formic acid is a weak acid
D) 100 ml
E) between 25 and 50 ml as formic acid is a weak acid
A) 50 ml
B) 25 ml
C) less than 25 ml as formic acid is a weak acid
D) 100 ml
E) between 25 and 50 ml as formic acid is a weak acid

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
The equivalence point for titration of 50 ml of 0.1 M formic acid, HCO2H (a weak acid) requires what volume of 0.2 M methylamine, CH3NH2 (a weak base)?
A) 50 ml
B) 25 ml
C) impossible to determine unless Ka for formic acid is known
D) impossible to determine unless Kb for methalamine is known
E) impossible to determine unless both Ka for formic acid and Kb for methylamine are known
A) 50 ml
B) 25 ml
C) impossible to determine unless Ka for formic acid is known
D) impossible to determine unless Kb for methalamine is known
E) impossible to determine unless both Ka for formic acid and Kb for methylamine are known

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
The equivalence point for titration of 50 ml of 0.1 M formic acid, HCO2H (a weak acid) requires what volume of 0.2 M calcium hydroxidem, Ca(OH)2?
A) 50 ml
B) 25 ml
C) less than 12.5 ml as formic acid is a weak acid
D) 12.5 ml
E) between 12.5 and 25 ml as formic acid is a weak acid
A) 50 ml
B) 25 ml
C) less than 12.5 ml as formic acid is a weak acid
D) 12.5 ml
E) between 12.5 and 25 ml as formic acid is a weak acid

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
Sodium carbonate, also called soda ash, is often analyzed by titration with strong acid. Initial concentrations of the carbonate ions are about 0.1 M. What indicator(s) would be suitable for detecting the stoichiometric point in this titration? (for H2CO3: pKa1 = 3.75 pKa2 = 10.33) 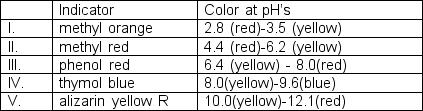
A) I only
B) I and II
C) III only
D) IV only
E) I and V

A) I only
B) I and II
C) III only
D) IV only
E) I and V

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
Your friend at Podunk U. urgently emails asking if you remember any acid-base chemistry. His problem is that for his final organic lab problem he needs to report the pKa of his acid. He did the pH titration, but only wrote down the following data for the titratant volumes: V = 14.27 mL; pH = 4.73. Vequivalence point = 22.43 mL. What is the pKa of the unknown acid?
A) 3.5
B) 4.0
C) 4.5
D) 4.8
E) 4.9
A) 3.5
B) 4.0
C) 4.5
D) 4.8
E) 4.9

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
The following graph shows the titration of a 0.150 M benzoic acid solution. At what pH range would benzoic acid be appropriate to make a buffer? 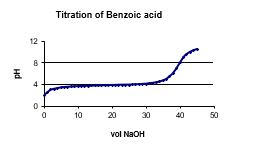
A) 2.2
B) 3.5
C) 4.0
D) 7.5
E) 10.0

A) 2.2
B) 3.5
C) 4.0
D) 7.5
E) 10.0

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
What is the solubility product expression for barium phosphate?
A) Ksp = [Ba2+][PO43-]
B) Ksp = [Ba2+]2[PO43-]3
C) Ksp = [Ba2+]3[PO43-]2
D) Ksp = [Ba2+]3[PO43-]3
E) Ksp = [Ba2+][PO43-]3
A) Ksp = [Ba2+][PO43-]
B) Ksp = [Ba2+]2[PO43-]3
C) Ksp = [Ba2+]3[PO43-]2
D) Ksp = [Ba2+]3[PO43-]3
E) Ksp = [Ba2+][PO43-]3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
What is the solubility product expression for iron (III) hydroxide?
A) Ksp = [Fe3+][OH-]
B) Ksp = [Fe3+][OH-]3
C) Ksp = [Fe3+]2[OH-]3
D) Ksp = [Fe3+]3[OH-]
E) Ksp = [Fe3+]3[OH-]3
A) Ksp = [Fe3+][OH-]
B) Ksp = [Fe3+][OH-]3
C) Ksp = [Fe3+]2[OH-]3
D) Ksp = [Fe3+]3[OH-]
E) Ksp = [Fe3+]3[OH-]3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
At what pH will an 0.0010 M of iron (III) nitrate (Ksp (Fe(OH)3)= 4.0 x 10-38) begin to precipitate?
A) 2.55
B) 4.22
C) 5.55
D) 7.41
E) 9.03
A) 2.55
B) 4.22
C) 5.55
D) 7.41
E) 9.03

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is NOT one of the equilibria in the complexation of Ru(II) by ammonia (coordination number = 6)?
A) Ru2+(aq) + NH3(aq) Ru(NH3)2+ (aq)
B) Ru2+(NH3)(aq) + NH3(aq) Ru2+(NH3)2 (aq)
C) Ru2+(NH3)4 (aq) + NH3(aq) Ru2+(NH3)5 (aq)
D) Ru2+(NH3)3 (aq) + NH3(aq) Ru2+(NH3)4 (aq)
E) Ru2+(NH3)6 (aq) + NH3(aq) Ru2+(NH3)7 (aq)
A) Ru2+(aq) + NH3(aq) Ru(NH3)2+ (aq)
B) Ru2+(NH3)(aq) + NH3(aq) Ru2+(NH3)2 (aq)
C) Ru2+(NH3)4 (aq) + NH3(aq) Ru2+(NH3)5 (aq)
D) Ru2+(NH3)3 (aq) + NH3(aq) Ru2+(NH3)4 (aq)
E) Ru2+(NH3)6 (aq) + NH3(aq) Ru2+(NH3)7 (aq)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
In which solution will copper nitrate (Cu(NO3)2) be the least soluble?
A) 0.1 M NaNO3
B) 0.1 M NH3
C) pure water
D) 0.1 M CuCl2
E) 0.1 M NaOH
A) 0.1 M NaNO3
B) 0.1 M NH3
C) pure water
D) 0.1 M CuCl2
E) 0.1 M NaOH

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
Write the equation showing how Na2HPO4 and NaH2PO4 act as a buffer. Also write an equation showing how this buffer reacts when HCl is added.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
When is a solution a buffer?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
What is the pH of a solution made by the addition of 0.34 mole of Na2HPO4 and 0.65 mole of NaH2PO4 and sufficient water to give a total volume of 1.2 L?If needed, use the following equation: pH = pKa + log(A-/HA)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
What is the pH upon adding 10 mL of 1.0 M NaOH toa) 1.2 L of water and b) a solution made by the addition of 0.34 mole of Na2HPO4 and 0.65 mole of NaH2PO4 and sufficient water to give a total volume of 1.2 L?If needed, use the following equation: pH = pKa + log(A-/HA)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
What is the pH of the resulting solution when 0.1 moles of HCl are added to 1 L of solution containing 0.12 moles of aqueous Na2CO3?If needed, use the following equation: pH = pKa + log(A-/HA)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
A solution is prepared by adding 0.14 mole Na2HPO4 and 8.2 grams of NaH2PO4 to sufficient water to prepare 1.00 L of solution. What is the pH of the solution? What is the pH of the solution after 0.041 moles HCl are added?If needed, use the following equation: pH = pKa + log(A-/HA)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
Determine the ratio of conjugate base to acid for a buffered solution at pH = 4.3 made from propionic acid, (Ka = 1.34 x10-5).

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
In the selective precipitation of metal ions as sulphide salts, usable concentrations of the S2- ions can be maintained by bubbling H2S through a solution buffered at a pH of about 10.0. How many mL of 6 M HCl would need to be added to 3.5 mL of a solution 5.6 M in aqueous ammonia to form a solution buffered at pH 10.0?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
A buffer is made by adding 0.82 moles of monohydrogen phosphate and 0.82 moles of dihydrogen phosphate to sufficient water to give a total volume of 1.0 L. Hydrogen chloride, 0.60 moles, is absorbed into the solution. What is the pH?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
A buffer solution made from ammonia, NH3, and ammonium chloride, NH4Cl, is prepared with pH 9.0. If Kb of NH3 is 1.78x10-5 and the total concentration of all nitrogen species is 0.25 M, what are the nitrogen concentrations?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
What mass of sodium acetate must be added to 250 mL of 0.10 M acetic acid (pKa = 4.75) to give a buffer of pH = 4.90?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
What mass of ammonium chloride (pKa (NH4+) = 9.25) must be added to 2.0 L of 0.65 M aqueous ammonia to give a buffer with pH = 8.75?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
How would you make a buffer of pH = 3.75 from the following solutions with a total volume of 300 ml? (Ka = 1.77 x10-4 for Formic Acid, HCO2H); 0.100M HCOOH; 0.100 M HClO4; 0.100 M NaOH; 0.100 M NH3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
How would you make a buffer of pH = 3.75 from the following solutions with a total volume of 450 ml? (Ka = 1.77 x10-4 for Formic Acid, HCO2H); 0.100M NaHCOO; 0.100 M HClO4; 0.100 M NaOH, 0.100 M NH3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the pH in the titration of a 0.325 g sample of acetylsalicylic acid (see line drawing below) initially in 25.0 mL water with 0.102 M NaOH when (a) 6.23 mL and (b) 20.00 mL of titrant have been added. 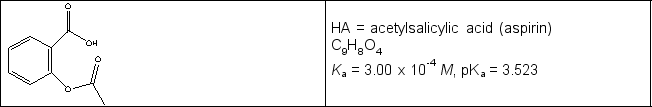


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the pH in the titration of a 0.325 g sample of acetylsalicylic acid (see line drawing below) initially in 25.0 mL water with 0.102 M NaOH when (a) 8.84 mL and (b) 17.68 mL of titrant have been added. 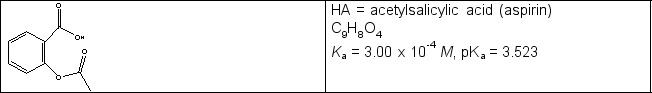


Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
A qualitative sketch of the titration curve for acetylsalicylic acid is shown at right.Given the indicators listed, which one would be suitable for titrations of acetylsalicylic acid? 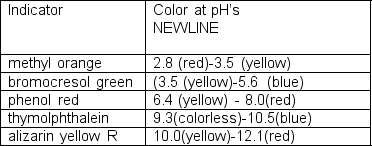
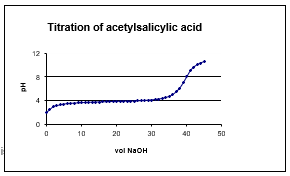



Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the titration of 25.0 mL of an 0.11 M lactic acid solution CH3CH(OH)COOH (Ka= 8.4 x10-4) with 0.150 M NaOH.a) Calculate the pH after 10 mL of the NaOH solution has been added.b) Calculate the pH of the solution at 10 mL of NaOH past the stoichiometric point.c) Would phenol red (pKin = 7.9) or phenolphthalein (pKin = 9.4) be a better indicator for this titration?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
A 5.00 mL sample containing sulphurous acid (Ka1 = 1.5 x 10-2; Ka2 = 1.0 x10-7) is titrated with 0.1324M NaOH. The stoichiometric point is at 25.32 mL. How many g of H2SO3 are present in the sample and what is the pH at the stoichiometric point?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
At what pH would a solution with a total concentration of 0.20 M phosphate NOT be a buffer? Explain your answer.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
What is the concentration of phosphate in a solution made from a 0.22 M phosphoric acid whose pH has been adjusted to 7.1 by the addition of solid potassium hydroxide (assume no volume change)?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
What is the concentration of phosphate in a solution made from a 0.15 M phosphoric acid whose pH has been adjusted to 7.6 by the addition of solid sodium hydroxide (assume no volume change)?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
An aqueous solution is 0.2 M in both Mg2+ and Pb2+ ions. You wish to separate the two metal ions by adding oxalate, C2O42-. What is the highest possible oxalate-ion concentration such that only one of the two metal ions will form a solid?Ksp (MgC2O4) = 8.5 x 10-5 Ksp (PbC2O4) = 2.7 x 10-11

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
An aqueous solution is 0.2 M in both Mg2+ and Pb2+ ions. You wish to separate the two metal ions by adding oxalate, C2O42-. What is the concentration of lead when Mg2+ begins to precipitate?Ksp (MgC2O4) = 8.5 x 10-5 Ksp (PbC2O4) = 2.7 x 10-11

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
100 ml of aqueous solution is 0.2 M in both Mg2+ and Pb2+ ions. You wish to separate the two metal ions by adding oxalate, C2O42-. What is the mass of PbC2O4 precipitated before Mg2+ begins to precipitate?Ksp (MgC2O4) = 8.5 x 10-5 Ksp (PbC2O4) = 2.7 x 10-11

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
At 100°C, 2.1 x 10-2 g of AgCl dissolves in 1.00 L of water. What is the Ksp of AgCl at 100°C?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
What is the concentration of Ca2+ in a saturated solution of calcium phosphate Ca3(PO4)2 (Ksp = 2.07 x 10-33)?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
A real world application of solubility products is found in softening "hard water" by removing calcium and magnesium ions present as the sulphate and carbonate salts. We can decrease the amount of Ca2+ in solution by addition of sodium carbonate, a strong electrolyte:Na2CO3 (s) 2 Na+ (aq) + CO32- (aq)By increasing the concentration of carbonate, we tend to drive the solubility equilibrium toward the reactant:CaCO3 (s) ![A real world application of solubility products is found in softening hard water by removing calcium and magnesium ions present as the sulphate and carbonate salts. We can decrease the amount of Ca<sup>2+</sup> in solution by addition of sodium carbonate, a strong electrolyte:Na<sub>2</sub>CO<sub>3</sub> (s) \rightarrow 2 Na<sup>+ </sup>(aq)<sup> </sup>+ CO<sub>3</sub><sup>2-</sup> (aq)By increasing the concentration of carbonate, we tend to drive the solubility equilibrium toward the reactant:CaCO<sub>3</sub> <sub>(</sub><sub>s</sub><sub>)</sub> Ca<sup>2+</sup> <sub>(</sub>aq<sub>)</sub> + CO<sub>3</sub><sup>2-</sup> (aq) K<sub>sp</sub> = 4.7 x 10<sup>-9</sup>If the initial [Ca<sup>2+</sup>] = 5.0 x 10<sup>-3</sup><sup> </sup>M, what percent of the [Ca<sup>2+</sup>] will be removed if the carbonate concentration is maintained at 1.0 x 10<sup>-3</sup> M?](https://storage.examlex.com/TB9687/11ee726d_d438_86de_827e_218b2c662730_TB9687_11.jpg) Ca2+ (aq) + CO32- (aq) Ksp = 4.7 x 10-9If the initial [Ca2+] = 5.0 x 10-3 M, what percent of the [Ca2+] will be removed if the carbonate concentration is maintained at 1.0 x 10-3 M?
Ca2+ (aq) + CO32- (aq) Ksp = 4.7 x 10-9If the initial [Ca2+] = 5.0 x 10-3 M, what percent of the [Ca2+] will be removed if the carbonate concentration is maintained at 1.0 x 10-3 M?
![A real world application of solubility products is found in softening hard water by removing calcium and magnesium ions present as the sulphate and carbonate salts. We can decrease the amount of Ca<sup>2+</sup> in solution by addition of sodium carbonate, a strong electrolyte:Na<sub>2</sub>CO<sub>3</sub> (s) \rightarrow 2 Na<sup>+ </sup>(aq)<sup> </sup>+ CO<sub>3</sub><sup>2-</sup> (aq)By increasing the concentration of carbonate, we tend to drive the solubility equilibrium toward the reactant:CaCO<sub>3</sub> <sub>(</sub><sub>s</sub><sub>)</sub> Ca<sup>2+</sup> <sub>(</sub>aq<sub>)</sub> + CO<sub>3</sub><sup>2-</sup> (aq) K<sub>sp</sub> = 4.7 x 10<sup>-9</sup>If the initial [Ca<sup>2+</sup>] = 5.0 x 10<sup>-3</sup><sup> </sup>M, what percent of the [Ca<sup>2+</sup>] will be removed if the carbonate concentration is maintained at 1.0 x 10<sup>-3</sup> M?](https://storage.examlex.com/TB9687/11ee726d_d438_86de_827e_218b2c662730_TB9687_11.jpg) Ca2+ (aq) + CO32- (aq) Ksp = 4.7 x 10-9If the initial [Ca2+] = 5.0 x 10-3 M, what percent of the [Ca2+] will be removed if the carbonate concentration is maintained at 1.0 x 10-3 M?
Ca2+ (aq) + CO32- (aq) Ksp = 4.7 x 10-9If the initial [Ca2+] = 5.0 x 10-3 M, what percent of the [Ca2+] will be removed if the carbonate concentration is maintained at 1.0 x 10-3 M? 
Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
How many grams of AgNO3 will dissolve in 1.32 L of a pH = 12.23 solution (Ksp AgOH = 1.52 x 10-8)?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
What is the molar concentration of barium in a 0.01 M ammonium sulphate solution saturated with Ba(NO3)2?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
A 1.32 g sample of solid CuCl2•2H2O is added to 1.21 L of 2.0 M aqueous ammonia. The formation constant for tetra-amminecopper (II) is 1.0 x 1012. What is the concentration of aqueous copper (II) in this solution?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
Trisbipyridylruthenium (II), Ru2+(BIPY)3, has been used in many studies, most recently to understand electrical conductivity in DNA molecules. Write all the equilibria in the formation of this complex.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
1.1 mg of HgS (Ksp1.6 x 10-54) is added to 1.0 L of 0.01 M NaCN ((Hg(CN)4+2), Kf = 4 x 1041)? What is the value of the reaction quotient for Qsp and will all of the HgS dissolve?

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Write the formation constant expression for the formation of [Zn(OH)4]-2 from Zn(OH)2(s).Zn(OH)2(s) + OH-(aq) ![Write the formation constant expression for the formation of [Zn(OH)<sub>4</sub>]<sup>-2</sup> from Zn(OH)<sub>2(s)</sub>.Zn(OH)<sub>2(s)</sub> + OH<sup>-</sup>(aq) [Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq)[Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq) + OH<sup>-</sup>(aq) [Zn(OH)<sub>4</sub>]<sup>-2</sup>(aq)](https://storage.examlex.com/TB9687/11ee726d_d438_fc12_827e_316527241e86_TB9687_11.jpg) [Zn(OH)3]-1(aq)[Zn(OH)3]-1(aq) + OH-(aq)
[Zn(OH)3]-1(aq)[Zn(OH)3]-1(aq) + OH-(aq) ![Write the formation constant expression for the formation of [Zn(OH)<sub>4</sub>]<sup>-2</sup> from Zn(OH)<sub>2(s)</sub>.Zn(OH)<sub>2(s)</sub> + OH<sup>-</sup>(aq) [Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq)[Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq) + OH<sup>-</sup>(aq) [Zn(OH)<sub>4</sub>]<sup>-2</sup>(aq)](https://storage.examlex.com/TB9687/11ee726d_d438_fc13_827e_15dfae61f4d8_TB9687_11.jpg) [Zn(OH)4]-2(aq)
[Zn(OH)4]-2(aq)
![Write the formation constant expression for the formation of [Zn(OH)<sub>4</sub>]<sup>-2</sup> from Zn(OH)<sub>2(s)</sub>.Zn(OH)<sub>2(s)</sub> + OH<sup>-</sup>(aq) [Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq)[Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq) + OH<sup>-</sup>(aq) [Zn(OH)<sub>4</sub>]<sup>-2</sup>(aq)](https://storage.examlex.com/TB9687/11ee726d_d438_fc12_827e_316527241e86_TB9687_11.jpg) [Zn(OH)3]-1(aq)[Zn(OH)3]-1(aq) + OH-(aq)
[Zn(OH)3]-1(aq)[Zn(OH)3]-1(aq) + OH-(aq) ![Write the formation constant expression for the formation of [Zn(OH)<sub>4</sub>]<sup>-2</sup> from Zn(OH)<sub>2(s)</sub>.Zn(OH)<sub>2(s)</sub> + OH<sup>-</sup>(aq) [Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq)[Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq) + OH<sup>-</sup>(aq) [Zn(OH)<sub>4</sub>]<sup>-2</sup>(aq)](https://storage.examlex.com/TB9687/11ee726d_d438_fc13_827e_15dfae61f4d8_TB9687_11.jpg) [Zn(OH)4]-2(aq)
[Zn(OH)4]-2(aq)
Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
Write the equilibrium constant expression and determine the solubility constant for CuS dissolving in an ammonia solution. (CuS, Ksp = 8 x10-37) (Cu(NH3)4+2, Kf = 1.1 x 1013)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
What is the concentration of [PbCl3]-prepared from a solution of 0.5 M CaCl2 and solid PbCl2?Pb2+ + 3Cl- ? [PbCl3]- Kf = 2.4 x 101pKsp(PbCl2) = 4.77

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
What is the concentration of [PbCl3]- when PbCl2 solid first begins to precipitate on addition of NaCl salt to a 0.5 M PbNO3 solution?
Pb2+ + 3Cl- [PbCl3]-
Kf = 2.4 x 101pKsp(PbCl2) = 4.77
Pb2+ + 3Cl- [PbCl3]-
Kf = 2.4 x 101pKsp(PbCl2) = 4.77

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
Write the equilibrium constant expression and determine the solubility constant for HgS dissolving in a cyanide solution. (HgS, Ksp = 1.6 x 10-54) ((Hg(CN)2), Kf = 4 x 1041)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck


