Exam 16: Applications of Aqueous Equilibria
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
Calculate the pH of a buffered solution.
Free
(Essay)
4.9/5  (43)
(43)
Correct Answer:
A buffer solution contains both a weak acid and its conjugate base, or a weak base and its conjugate acid, as major species in solution.
Determine the ratio of conjugate base to acid for a buffered solution at pH = 4.3 made from propionic acid, (Ka = 1.34 x10-5).
Free
(Short Answer)
4.9/5  (35)
(35)
Correct Answer:
A-/HA = 0.267
In which of the following titrations will the pH be less than 7 at the equivalence point?
Free
(Multiple Choice)
4.8/5  (49)
(49)
Correct Answer:
E
Which of the following will have the smallest pH change upon the addition of 0.001 mole strong acid?
(Multiple Choice)
4.8/5  (31)
(31)
What is the pH of a solution made by the addition of 0.34 mole of Na2HPO4 and 0.65 mole of NaH2PO4 and sufficient water to give a total volume of 1.2 L?If needed, use the following equation: pH = pKa + log(A-/HA)
(Short Answer)
4.8/5  (39)
(39)
A biochemist wishes to study the activity of an enzyme that produces one mole hydrogen ion for every mole of product produced. However, the enzyme is sensitive to changes in pH and will become inactive at pH less than 7.1. If the initial pH of the solution is 7.21 set with a phosphate buffer (the pKa's of the three acid-conjugate base pairs derived from phosphoric acid, H3PO4, are 2.12, 7.21 and 12.32) and the reaction is designed to produce 0.15 moles of product in a volume of 250 mL, what is the minimum concentration of the conjugate acid in the buffer solution?
(Multiple Choice)
4.9/5  (33)
(33)
What is the concentration of Ca2+ in a saturated solution of calcium phosphate Ca3(PO4)2 (Ksp = 2.07 x 10-33)?
(Short Answer)
4.9/5  (31)
(31)
An aqueous solution is 0.2 M in both Mg2+ and Pb2+ ions. You wish to separate the two metal ions by adding oxalate, C2O42-. What is the highest possible oxalate-ion concentration such that only one of the two metal ions will form a solid?Ksp (MgC2O4) = 8.5 x 10-5 Ksp (PbC2O4) = 2.7 x 10-11
(Short Answer)
4.7/5  (33)
(33)
How would you make a buffer of pH = 3.75 from the following solutions with a total volume of 300 ml? (Ka = 1.77 x10-4 for Formic Acid, HCO2H); 0.100M HCOOH; 0.100 M HClO4; 0.100 M NaOH; 0.100 M NH3
(Short Answer)
4.8/5  (29)
(29)
The equivalence point for titration of 50 ml of 0.1 M formic acid, HCO2H (a weak acid) requires what volume of 0.2 M calcium hydroxidem, Ca(OH)2?
(Multiple Choice)
5.0/5  (32)
(32)
A 5.00 mL sample containing sulphurous acid (Ka1 = 1.5 x 10-2; Ka2 = 1.0 x10-7) is titrated with 0.1324M NaOH. The stoichiometric point is at 25.32 mL. How many g of H2SO3 are present in the sample and what is the pH at the stoichiometric point?
(Short Answer)
4.9/5  (34)
(34)
When 0.1 moles of HCl are added to 1 L of solution containing 0.12 moles of aqueous Na2CO3, the major species present are
(Multiple Choice)
4.9/5  (38)
(38)
When 0.1 moles of HC2H3O2 and 0.1 moles of NaC2H3O2 are added to make 1 L of aqueous solution, the major species present are
(Multiple Choice)
4.9/5  (45)
(45)
What is the concentration of phosphate in a solution made from a 0.22 M phosphoric acid whose pH has been adjusted to 7.1 by the addition of solid potassium hydroxide (assume no volume change)?
(Short Answer)
4.9/5  (32)
(32)
A buffer solution made from ammonia, NH3, and ammonium chloride, NH4Cl, is prepared with pH 9.0. If Kb of NH3 is 1.78x10-5 and the total concentration of all nitrogen species is 0.25 M, what are the nitrogen concentrations?
(Short Answer)
4.9/5  (42)
(42)
In the selective precipitation of metal ions as sulphide salts, usable concentrations of the S2- ions can be maintained by bubbling H2S through a solution buffered at a pH of about 10.0. How many mL of 6 M HCl would need to be added to 3.5 mL of a solution 5.6 M in aqueous ammonia to form a solution buffered at pH 10.0?
(Short Answer)
4.8/5  (35)
(35)
The following graph shows the titration of a 0.150 M benzoic acid solution. At what pH range would benzoic acid be appropriate to make a buffer? 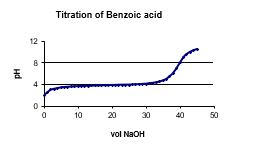
(Multiple Choice)
4.9/5  (33)
(33)
Write the equilibrium constant expression and determine the solubility constant for HgS dissolving in a cyanide solution. (HgS, Ksp = 1.6 x 10-54) ((Hg(CN)2), Kf = 4 x 1041)
(Essay)
4.8/5  (29)
(29)
What is the concentration of [PbCl3]- when PbCl2 solid first begins to precipitate on addition of NaCl salt to a 0.5 M PbNO3 solution?
Pb2+ + 3Cl- [PbCl3]-
Kf = 2.4 x 101pKsp(PbCl2) = 4.77
(Short Answer)
4.9/5  (44)
(44)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)