Deck 9: Properties of Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 9: Properties of Solutions
1
Work with and interconvert concentration units (mass %, molarity, molality, mole fraction, ppm, and ppb).
The solvent determines the phase of the solution. Concentrations are expressed as amount of solute divided by the amount of solvent, or by the amount of solution.
2
Predict the relative solubilities of a solute in various solvents, and explain in terms of intermolecular forces.
Substances that dissolve in each other usually have similar types of intermolecular interactions ("like dissolves like").
3
Calculate and explain solubilities in water.
Enthalpies of solution and of dilution depend on the lattice energy of the salt as well as the hydration energies of its ions. The solubility of a gas in a liquid depends on the partial pressure of the gas.
4
Understand the reasons for and calculate the magnitudes of colligative properties.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Understand some aspects of colloidal suspensions and surfactant solutions.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
What is the molarity of a solution made from mixing 34 grams of CaCl2 with enough water to make 150 ml of solution?
A) 2.04 M
B) 1.02 M
C) 3.60 M
D) 0.20 M
E) 0.34 M
A) 2.04 M
B) 1.02 M
C) 3.60 M
D) 0.20 M
E) 0.34 M

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
What is the molarity of an aqueous solution that is 0.569 m CaCl2 (solution density 1.05 g/ml)?
A) 0.569 M
B) 0.562 M
C) 0.597 M
D) 0.507 M
E) 0.507 m
A) 0.569 M
B) 0.562 M
C) 0.597 M
D) 0.507 M
E) 0.507 m

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
What is the mass percent of an aqueous solution that is 0.569 m CaCl2 (solution density 1.05 g/ml)?
A) 5.94 %
B) 6.32 %
C) 0.06 %
D) 5.94 x 10-2 %
E) 6.32 x10-2%
A) 5.94 %
B) 6.32 %
C) 0.06 %
D) 5.94 x 10-2 %
E) 6.32 x10-2%

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
What is the mole fraction of CaCl2 in an aqueous solution that is 0.569 m CaCl2 (solution density 1.05 g/ml)?
A) 0.01 M
B) 0.01
C) 0.57
D) 0.10
E) 9.6 x 10-3
A) 0.01 M
B) 0.01
C) 0.57
D) 0.10
E) 9.6 x 10-3

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
What is the molality of a radiator solution made by mixing equal volumes of ethylene glycol C2H6O2, (density = 1.11 g/ml) and water?
A) 17.9 m
B) 11.1 m
C) 1.79 m
D) 4.60 m
E) 17.9 M
A) 17.9 m
B) 11.1 m
C) 1.79 m
D) 4.60 m
E) 17.9 M

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
What is the molality of a solution made from mixing 39 grams of sugar (MM = 180 g/mol) with 310 grams of water?
A) 0.70 m
B) 1.40 m
C) 0.39 m
D) 3.10 m
E) 0.39 M
A) 0.70 m
B) 1.40 m
C) 0.39 m
D) 3.10 m
E) 0.39 M

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
What is the mole fraction of ethyl alcohol (C2H6O, density = 0.79 g/ml) in water for a solution made from equal volumes of each?
A) 0.235
B) 0.122
C) 0.756
D) 1.11
E) 17.9
A) 0.235
B) 0.122
C) 0.756
D) 1.11
E) 17.9

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
Why was the concentration unit of molality developed?
A) Molarity had not been developed yet.
B) Morality had not been developed yet.
C) Solution volumes changed with temperature.
D) Solution volumes changed with pressure.
E) Solution masses changed upon mixing.
A) Molarity had not been developed yet.
B) Morality had not been developed yet.
C) Solution volumes changed with temperature.
D) Solution volumes changed with pressure.
E) Solution masses changed upon mixing.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
The interactions that play an important role in the dissolution of a salt in water are
I. ion-dipole
II. hydrogen bonding
III. dipole-dipole
IV. ion-ion
A) I only
B) II and I
C) I and IV
D) II, III, and IV
E) I, II and IV
I. ion-dipole
II. hydrogen bonding
III. dipole-dipole
IV. ion-ion
A) I only
B) II and I
C) I and IV
D) II, III, and IV
E) I, II and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
Benzene and water are immiscible, forming two separate layers when mixed. Samples of the compounds below are shaken in a mixture of benzene and water. Which of the following compounds would you expect to be found in the benzene layer? 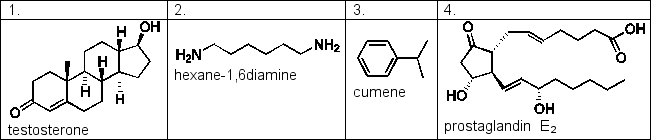
A) 2 and 4
B) 2 and 3
C) 1 and 4
D) 1 and 3
E) 1, 3 and 4

A) 2 and 4
B) 2 and 3
C) 1 and 4
D) 1 and 3
E) 1, 3 and 4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
List the following in order of solubility in hexane:
1) CH3CH2C(O)CH3,
2) C6H6,
3) CH3CH2CH2CO2H,
4) CH3CH2CH2CH2CO2H
A) 2 < 1 < 3 < 4
B) 1 < 3 < 4 < 2
C) 3 < 4 < 1 < 2
D) 4 < 3 < 1 < 2
E) 1 < 2 < 3 < 4
1) CH3CH2C(O)CH3,
2) C6H6,
3) CH3CH2CH2CO2H,
4) CH3CH2CH2CH2CO2H
A) 2 < 1 < 3 < 4
B) 1 < 3 < 4 < 2
C) 3 < 4 < 1 < 2
D) 4 < 3 < 1 < 2
E) 1 < 2 < 3 < 4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
How can mercury be used in dental fillings?
A) It is a solid at body temperature.
B) It forms an amalgam with your tooth enamel.
C) It forms an amalgam with other metals.
D) It is an insoluble salt in your mouth.
E) It forms a compound called brass with copper.
A) It is a solid at body temperature.
B) It forms an amalgam with your tooth enamel.
C) It forms an amalgam with other metals.
D) It is an insoluble salt in your mouth.
E) It forms a compound called brass with copper.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements regarding alloys is(are) true?
I. Alloys are homogeneous solid solutions
II. Alloys display metallic properties
III. The solute component in the alloy can replace the solvent component in the solid crystalline structure
IV. The solute component can be incorporated in between solvent atoms in the crystal structure
A) I only
B) II and I
C) I and IV
D) II, III, and IV
E) I, II and IV
I. Alloys are homogeneous solid solutions
II. Alloys display metallic properties
III. The solute component in the alloy can replace the solvent component in the solid crystalline structure
IV. The solute component can be incorporated in between solvent atoms in the crystal structure
A) I only
B) II and I
C) I and IV
D) II, III, and IV
E) I, II and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
What is the concentration of chloride ions in a solution that is 0.569 m CaCl2 (solution density 1.05 g/ml)?
A) 0.569 M
B) 0.562M
C) 1.124 M
D) 0.507 M
E) 1.138 M
A) 0.569 M
B) 0.562M
C) 1.124 M
D) 0.507 M
E) 1.138 M

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
What is the concentration of all ions in a solution that is 0.569 m CaCl2 (solution density 1.05 g/ml)?
A) 0.569 M
B) 0.562M
C) 1.124 M
D) 1.686 M
E) 2.248 M
A) 0.569 M
B) 0.562M
C) 1.124 M
D) 1.686 M
E) 2.248 M

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
The greatest gas solubility for a gas in solution is predicted under what conditions?
A) low T, low P
B) low T, high P
C) high T, low P
D) high T, high P
E) Solubility of gases does not depend upon temperature.
A) low T, low P
B) low T, high P
C) high T, low P
D) high T, high P
E) Solubility of gases does not depend upon temperature.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
Consider a warm (30°) 355 mL can of soda, under 2.5 atm CO2 pressure. What volume of CO2 will be released from the soda in order to reach equilibrium after opening to the room where the pressure of CO2 = 3.25 x 10-4 atm and the total pressure is 758 mm Hg (KH = 1.6 x 10-2 M/atm)?
A) 3.5 mL
B) 3.5 L
C) 35 mL
D) 0.35 L
E) 100 mL
A) 3.5 mL
B) 3.5 L
C) 35 mL
D) 0.35 L
E) 100 mL

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
According to Henry's Law, the solubility of a gas in a liquid
A) depends on the polarity of the liquid.
B) depends on the liquid's density.
C) remains the same at all temperatures.
D) increases as the gas pressure above the solution increases.
E) decreases as the gas pressure above the solution increases.
A) depends on the polarity of the liquid.
B) depends on the liquid's density.
C) remains the same at all temperatures.
D) increases as the gas pressure above the solution increases.
E) decreases as the gas pressure above the solution increases.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
Carbon dioxide and O2 are non-polar molecules. Can you provide some rational why CO2 dissolves so much more in water than O2?
A) CO2 can hydrogen bond and O2 cannot.
B) CO2 reacts with water and O2 does not.
C) O2 reacts with water and CO2 does not.
D) CO2 forms a solid with water.
E) Water decomposes to O2.
A) CO2 can hydrogen bond and O2 cannot.
B) CO2 reacts with water and O2 does not.
C) O2 reacts with water and CO2 does not.
D) CO2 forms a solid with water.
E) Water decomposes to O2.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following has the substances arranged in order of increasing heat of fusion?
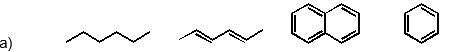

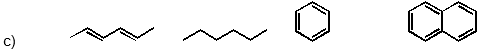

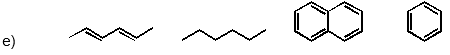






Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following statements regarding solubility as a function of temperature are correct?
I. Gas solubility favours high temperature and high pressure.
II. Gas solubility favours low temperature and high pressure.
III. Since the dissolution of solids into liquids is exothermic, solid solubility favours high temperature.
IV. Endothermic dissolution of solids in liquids is favoured by lower temperature.
V. Endothermic dissolution of solids in liquids is favoured by higher temperature.
A) I and V
B) II and IV
C) II and III
D) II and V
E) I and IV
I. Gas solubility favours high temperature and high pressure.
II. Gas solubility favours low temperature and high pressure.
III. Since the dissolution of solids into liquids is exothermic, solid solubility favours high temperature.
IV. Endothermic dissolution of solids in liquids is favoured by lower temperature.
V. Endothermic dissolution of solids in liquids is favoured by higher temperature.
A) I and V
B) II and IV
C) II and III
D) II and V
E) I and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
The vapour pressure of toluene at 25oC is 3.79 KPa and that of benzene is 12.7 kPa. The vapour above a solution that has mole fraction of benzene = 0.5,
A) has mole fraction toluene in 0.5.
B) has mole fraction of toluene less than 0.5.
C) has mole fraction of toluene greater than 0.5.
D) is pure benzene.
E) is pure toluene.
A) has mole fraction toluene in 0.5.
B) has mole fraction of toluene less than 0.5.
C) has mole fraction of toluene greater than 0.5.
D) is pure benzene.
E) is pure toluene.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
The boiling point of toluene is 110oC and that of benzene is 80oC. The vapour above a solution that has mole fraction of benzene = 0.5,
A) has mole fraction toluene in 0.5.
B) has mole fraction of toluene less than 0.5.
C) has mole fraction of toluene greater than 0.5.
D) is pure benzene.
E) is pure toluene.
A) has mole fraction toluene in 0.5.
B) has mole fraction of toluene less than 0.5.
C) has mole fraction of toluene greater than 0.5.
D) is pure benzene.
E) is pure toluene.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
Sea water has about 0.46 moles NaCl and 0.065 moles of MgCl2 in every litre. What is the vapour pressure of sea water at 30?C if pure water would have a vapour pressure of 33.2 mm Hg? (Assume there are 1000 grams of water in 1 litre of sea water.)
A) 33.2 mm Hg
B) 32.5 mm Hg
C) 37.0 mm Hg
D) 30.3 mm Hg
E) 32.9 mm Hg
A) 33.2 mm Hg
B) 32.5 mm Hg
C) 37.0 mm Hg
D) 30.3 mm Hg
E) 32.9 mm Hg

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
Which aqueous solution would be expected to have the highest boiling point?
A) 0.10 M NaCl
B) 0.080 M CaCl2
C) 0.064 M Fe(NO3)3
D) 0.081 M Fe(NO3)2
E) 0.080 M Co(SO4)
A) 0.10 M NaCl
B) 0.080 M CaCl2
C) 0.064 M Fe(NO3)3
D) 0.081 M Fe(NO3)2
E) 0.080 M Co(SO4)

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
In the textbook it was discussed how the melting point is decreased and the boiling point is elevated for a solvent with a solute in it. What happens to the triple point of that solvent? Does the triple point
A) remain at the same pressure and temperature?
B) stay at the same pressure, but warmer temperature?
C) stay at the same pressure, but cooler temperature?
D) occur at a lower pressure and temperature?
E) occur at a higher pressure and temperature?
A) remain at the same pressure and temperature?
B) stay at the same pressure, but warmer temperature?
C) stay at the same pressure, but cooler temperature?
D) occur at a lower pressure and temperature?
E) occur at a higher pressure and temperature?

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
Solutions used for intravenous injections must be isotonic, that is, exert an osmotic pressure of 6.9 atm. An isotonic saline solution uses sodium chloride as the solute to achieve isotonic conditions. Use this value for the osmotic pressure to estimate the % NaCl by mass in an isotonic saline solution at 37°C (assume the solution has a density of 1.0 g/mL).
A) 0.40%
B) 0.50%
C) 0.60%
D) 0.80%
E) 0.90%
A) 0.40%
B) 0.50%
C) 0.60%
D) 0.80%
E) 0.90%

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
A major component of cell membranes is the phospholipid bilayer. Which of the following statements is(are) FALSE?
I. The bilayer provides a barrier to charged ions
II. Polar molecules such as glucose can pass through the membrane
III. Protein molecules act as gates in the phospholipid bilayer allowing nutrients to enter into the cell
IV. The bilayer consists of two nearly parallel rows with the hydrophobic tails facing out toward the solution and acting as a barrier to water soluble species.
A) all
B) II and I
C) I and III
D) II and IV
E) III and IV
I. The bilayer provides a barrier to charged ions
II. Polar molecules such as glucose can pass through the membrane
III. Protein molecules act as gates in the phospholipid bilayer allowing nutrients to enter into the cell
IV. The bilayer consists of two nearly parallel rows with the hydrophobic tails facing out toward the solution and acting as a barrier to water soluble species.
A) all
B) II and I
C) I and III
D) II and IV
E) III and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following molecules would be suitable surfactants?
1)
2)
3)
4)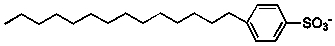
A) all
B) 1 and 2
C) 1 and 3
D) 2 and 4
E) 1 and 4
1)

2)

3)

4)

A) all
B) 1 and 2
C) 1 and 3
D) 2 and 4
E) 1 and 4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following are true solutions: milk, brass, NaCl in water, sugar in water, ink?
A) milk, NaCl in water, sugar in water
B) milk, brass, NaCl in water, sugar in water
C) NaCl in water, sugar in water, ink
D) brass, NaCl in water, sugar in water
E) NaCl in water, sugar in water
A) milk, NaCl in water, sugar in water
B) milk, brass, NaCl in water, sugar in water
C) NaCl in water, sugar in water, ink
D) brass, NaCl in water, sugar in water
E) NaCl in water, sugar in water

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following are colloidal suspensions: fog, milk, sugar in water, salt in water, styrofoam?
A) fog and milk
B) milk, sugar in water, styrofoam
C) fog, sugar in water styrofoam
D) fog, milk, styrofoam
E) fog and styrofoam
A) fog and milk
B) milk, sugar in water, styrofoam
C) fog, sugar in water styrofoam
D) fog, milk, styrofoam
E) fog and styrofoam

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
A mixture that does NOT settle out upon standing is
A) solid.
B) a suspension.
C) tyndall solution.
D) a colloid.
E) hydrated.
A) solid.
B) a suspension.
C) tyndall solution.
D) a colloid.
E) hydrated.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
Classify the following molecules or fragments as hydrophobic or hydrophilic. A line from an atom indicates where a bond would be formed to another fragment.
1. the -CH2SO3- group
2. CH3CH2OH
3 the -CH2(CH2)11CH3 group
4. the -NH3+ group
A) 1; hydrophilic: 2; hydrophilic: 3; hydrophilic: 4; hydrophobic
B) 1; hydrophilic: 2; hydrophobic: 3; hydrophilic: 4; hydrophobic
C) 1; hydrophilic: 2; hydrophilic: 3; hydrophobic: 4; hydrophilic
D) 1; hydrophilic: 2; hydrophobic: 3; hydrophilic: 4; hydrophilic
E) 1; hydrophobic: 2; hydrophilic: 3; hydrophilic: 4; hydrophilic
1. the -CH2SO3- group
2. CH3CH2OH
3 the -CH2(CH2)11CH3 group
4. the -NH3+ group
A) 1; hydrophilic: 2; hydrophilic: 3; hydrophilic: 4; hydrophobic
B) 1; hydrophilic: 2; hydrophobic: 3; hydrophilic: 4; hydrophobic
C) 1; hydrophilic: 2; hydrophilic: 3; hydrophobic: 4; hydrophilic
D) 1; hydrophilic: 2; hydrophobic: 3; hydrophilic: 4; hydrophilic
E) 1; hydrophobic: 2; hydrophilic: 3; hydrophilic: 4; hydrophilic

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
Which part of soap is responsible for its ability to mix with oily dirt?
A) the carboxylate end
B) the salt end
C) the hydrophilic end
D) the hydrophobic end
E) the ionized "head"
A) the carboxylate end
B) the salt end
C) the hydrophilic end
D) the hydrophobic end
E) the ionized "head"

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
Pesticides are non-polar molecules in general. What needs to be done so that farmers are able to spray aqueous solutions containing pesticides?
A) The solutions need to be heated.
B) An emulsifier is added.
C) A miscible agent is added.
D) The solution is shaken frequently.
E) Pesticides will never make a solution with water.
A) The solutions need to be heated.
B) An emulsifier is added.
C) A miscible agent is added.
D) The solution is shaken frequently.
E) Pesticides will never make a solution with water.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
A solution is made from dissolving 102 grams of potassium carbonate in 100 grams of water. What is the solvent in this solute in the solution and explain.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
Determine the mole fraction of a solution made from mixing 39 grams of sugar (MM = 180 g/mol) with 310 grams of water.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
Industrial grade concentrated HCl is known as muriatic acid and is 31.45% by mass HCl in water. What is the molality of this solution?

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
The molecule drawn below is Vitamin D, Calciferol. Would you predict this molecule to be more soluble in water or body fat and explain why? 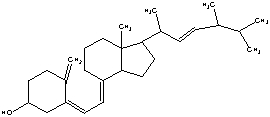


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
The molecule drawn below is Vitamin B1, thiamine. Would you predict this molecule to be more soluble in water or body fat and explain why? 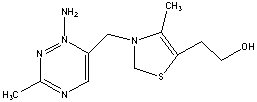


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
Pesticides have been found to accumulate and affect the growth of animals higher in food chain. Can you give some rational for this, including discussion of the solubility of pesticides in general?

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
When both solid Na metal and solid NaCl are added to water a colourless solution develops. How are these processes different?

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
Consider a can containing 355 mL of a carbonated soft drink at 0°C under 2.2 atm CO2 pressure. What is the concentration of CO2 in the soda, assuming that KH = 7.8 x 10-2 M/atm?

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
The problem with hot water heaters in some parts of the country is that a solid forms inside of them, CaCO3. Predict if the solvation process for CaCO3 is endothermic or exothermic and if it is more soluble or less soluble at colder temperatures.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
Fahrenheit developed his temperature scale where he set zero degrees as the freezing point for a salt-water solution. What is the concentration of this salt solution in molality (assume NaCl in water)? (Tf = 1.86 ˚C/m)

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
Automobile coolant is about 50% by mass ethylene glycol in water. The line structure of ethylene glycol is shown below:  What is the normal boiling point of this solution if Kb for water = 0.512 ˚Cm-1?
What is the normal boiling point of this solution if Kb for water = 0.512 ˚Cm-1?
 What is the normal boiling point of this solution if Kb for water = 0.512 ˚Cm-1?
What is the normal boiling point of this solution if Kb for water = 0.512 ˚Cm-1?
Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
If you have ever made homemade ice cream you would know that you need to add salt to the ice surrounding the container that you make the ice cream in. By adding the salt to the ice it lowers the temperature of the ice. Explain what is happening to the salt when added to the ice and what you can say about the heat of solvation for sodium chloride.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
When 0.64 g of adrenaline is dissolved in 36.0 g benzene (Kf = 5.12 °C), the freezing point is lowered by 0.50°C. Determine the molecular mass of adrenaline.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
A 7.85 g sample of a compound with an empirical formula C1H1 is dissolved in 172 g of benzene. The freezing point of the solution is 1.50 C below that of pure benzene. Assuming that the compound does not dissociate, what is the molar mass and molecular formula of this compound? The freezing point depression constant for benzene is Kf = 5.12 C/m.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
To what minimum temperature would a radiator be protected if equal volumes of ethylene glycol (permanent antifreeze) (density 1.11 g/mL) and water were mixed? Assume that ethylene glycol does not dissociate and has a formula, C2H6O2. (Kf = 1.85 ˚Cm-1).

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
What is the freezing point of a solution made from mixing 39 grams of sugar (MM = 180 g/mol) with 310 grams of water? (Kf = 1.85 ˚Cm-1)?

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
Lysozyme is an enzyme found in tears whose function is to break down bacterial cell walls thus killing the bacteria and protecting the eye from infection. A solution made which contains 0.100 g of lysozyme in 150 g of water ( = 1.00 g/mL) at 25°C has an osmotic pressure of 8.9 Torr. What is the molar mass of this enzyme?

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
Draw a molecular picture of a surfactant on a waxed surface, like soapy water on a freshly waxed car.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
Ben Franklin determined Avogadro's number within a factor of 5. He was able to do this by dropping 1 teaspoon of oil on a still pond and determined the area covered by the oil at about ¾ of an acre. You can simulate this by adding 1 drop of soap on the top of a greasy pan in the kitchen and watch the grease move to the sides. Sketch the interaction of the soap with the layer of water and sketch how the soap "dissolves" grease in water.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


