Exam 9: Properties of Solutions
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
The molecule drawn below is Vitamin D, Calciferol. Would you predict this molecule to be more soluble in water or body fat and explain why? 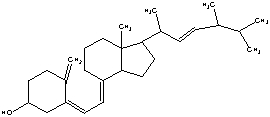
Free
(Essay)
4.7/5  (42)
(42)
Correct Answer:
More soluble in fat, as it has only one small polar group (OH). The rest of the molecule is non-polar and would therefore more readily dissolve in fat.
Sea water has about 0.46 moles NaCl and 0.065 moles of MgCl2 in every litre. What is the vapour pressure of sea water at 30?C if pure water would have a vapour pressure of 33.2 mm Hg? (Assume there are 1000 grams of water in 1 litre of sea water.)
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
B
Benzene and water are immiscible, forming two separate layers when mixed. Samples of the compounds below are shaken in a mixture of benzene and water. Which of the following compounds would you expect to be found in the benzene layer? 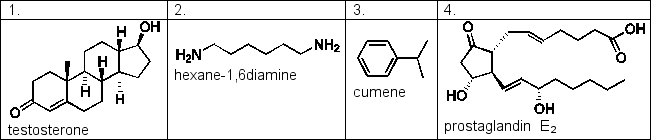
Free
(Multiple Choice)
5.0/5  (36)
(36)
Correct Answer:
D
Automobile coolant is about 50% by mass ethylene glycol in water. The line structure of ethylene glycol is shown below:  What is the normal boiling point of this solution if Kb for water = 0.512 ˚Cm-1?
What is the normal boiling point of this solution if Kb for water = 0.512 ˚Cm-1?
(Short Answer)
4.8/5  (32)
(32)
What is the mass percent of an aqueous solution that is 0.569 m CaCl2 (solution density 1.05 g/ml)?
(Multiple Choice)
4.9/5  (43)
(43)
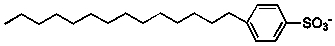
Which of the following molecules would be suitable surfactants?
1)  2)
2)  3)
3)  4)
4) 
(Multiple Choice)
4.9/5  (33)
(33)
What is the freezing point of a solution made from mixing 39 grams of sugar (MM = 180 g/mol) with 310 grams of water? (Kf = 1.85 ˚Cm-1)?
(Short Answer)
4.7/5  (34)
(34)
What is the mole fraction of ethyl alcohol (C2H6O, density = 0.79 g/ml) in water for a solution made from equal volumes of each?
(Multiple Choice)
4.9/5  (35)
(35)
Determine the mole fraction of a solution made from mixing 39 grams of sugar (MM = 180 g/mol) with 310 grams of water.
(Short Answer)
4.9/5  (42)
(42)
When both solid Na metal and solid NaCl are added to water a colourless solution develops. How are these processes different?
(Essay)
4.9/5  (35)
(35)
What is the molality of a radiator solution made by mixing equal volumes of ethylene glycol C2H6O2, (density = 1.11 g/ml) and water?
(Multiple Choice)
4.8/5  (40)
(40)
Work with and interconvert concentration units (mass %, molarity, molality, mole fraction, ppm, and ppb).
(Essay)
4.8/5  (33)
(33)
A 7.85 g sample of a compound with an empirical formula C1H1 is dissolved in 172 g of benzene. The freezing point of the solution is 1.50 C below that of pure benzene. Assuming that the compound does not dissociate, what is the molar mass and molecular formula of this compound? The freezing point depression constant for benzene is Kf = 5.12 C/m.
(Short Answer)
4.9/5  (41)
(41)
The boiling point of toluene is 110oC and that of benzene is 80oC. The vapour above a solution that has mole fraction of benzene = 0.5,
(Multiple Choice)
4.8/5  (39)
(39)
Fahrenheit developed his temperature scale where he set zero degrees as the freezing point for a salt-water solution. What is the concentration of this salt solution in molality (assume NaCl in water)? (Tf = 1.86 ˚C/m)
(Essay)
4.9/5  (32)
(32)
If you have ever made homemade ice cream you would know that you need to add salt to the ice surrounding the container that you make the ice cream in. By adding the salt to the ice it lowers the temperature of the ice. Explain what is happening to the salt when added to the ice and what you can say about the heat of solvation for sodium chloride.
(Essay)
5.0/5  (45)
(45)
Which of the following are colloidal suspensions: fog, milk, sugar in water, salt in water, styrofoam?
(Multiple Choice)
4.8/5  (38)
(38)
Carbon dioxide and O2 are non-polar molecules. Can you provide some rational why CO2 dissolves so much more in water than O2?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 59
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)