Deck 4: Protein Structure and Folding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/63
Play
Full screen (f)
Deck 4: Protein Structure and Folding
1
The following is a short segment of an mRNA molecule. The polypeptide it codes for is also shown:
5 ' AUGGUGCUGAAG 3 ': methionine-valine-leucine-lysine
A mutation in the DNA occurs so that the fourth base (counting from the 5 ' end) in the messenger RNA now reads A rather than G. What sequence of peptides will the mRNA now code for? (You do not need a copy of the genetic code to answer the question.)
A) methionine-lysine-leucine-lysine
B) methionine-leucine-leucine-lysine
C) methionine-valine-methionine-lysine
D) methionine-methionine-leucine-lysine
5 ' AUGGUGCUGAAG 3 ': methionine-valine-leucine-lysine
A mutation in the DNA occurs so that the fourth base (counting from the 5 ' end) in the messenger RNA now reads A rather than G. What sequence of peptides will the mRNA now code for? (You do not need a copy of the genetic code to answer the question.)
A) methionine-lysine-leucine-lysine
B) methionine-leucine-leucine-lysine
C) methionine-valine-methionine-lysine
D) methionine-methionine-leucine-lysine
D
2
The genetic code is said to be "degenerate" because
A) More than one type of tRNA can be charged with a particular amino acid.
B) A single tRNA specific for a particular amino acid may respond to multiple codons in an mRNA
C) A single tRNA may couple a single mRNA codon to multiple amino acids.
D) A single codon in an mRNA can couple to the anticodons of many different types of tRNAs.
A) More than one type of tRNA can be charged with a particular amino acid.
B) A single tRNA specific for a particular amino acid may respond to multiple codons in an mRNA
C) A single tRNA may couple a single mRNA codon to multiple amino acids.
D) A single codon in an mRNA can couple to the anticodons of many different types of tRNAs.
A single tRNA specific for a particular amino acid may respond to multiple codons in an mRNA
3
Which statement about the genetic code is NOT correct?
A) Non-Watson-Crick base pairing can occur at the third position of codons and anticodons.
B) The sense strand of DNA serves as the template for RNA synthesis.
C) A particular amino acid may be coded for by more than one codon.
D) A DNA sequence is read in triplets.
A) Non-Watson-Crick base pairing can occur at the third position of codons and anticodons.
B) The sense strand of DNA serves as the template for RNA synthesis.
C) A particular amino acid may be coded for by more than one codon.
D) A DNA sequence is read in triplets.
The sense strand of DNA serves as the template for RNA synthesis.
4
_____________ different genetically-encoded amino acids are found in the proteins of cells; they are distinguished by___________________ .
A) 22; the location of their carboxyl group
B) 20; the location of their amino group
C) 20; the angles between the central carbon and their side chains, or R-groups
D) 22; the composition of their side chains, or R-groups
A) 22; the location of their carboxyl group
B) 20; the location of their amino group
C) 20; the angles between the central carbon and their side chains, or R-groups
D) 22; the composition of their side chains, or R-groups

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following are rare amino acids that are sometimes encoded by stop codons?
A) isoleucine and pyrroglutamine
B) selenocysteine and pyrrolysine
C) pseudouridine and D-alanine
D) abrine and homocysteine
A) isoleucine and pyrroglutamine
B) selenocysteine and pyrrolysine
C) pseudouridine and D-alanine
D) abrine and homocysteine

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
6
The modified nucleotide inosine (I) found in tRNA can pair with
A) the base A only
B) any of the three bases, U, C, and A
C) either U or A
D) any of the four bases, U, C, G, and A
A) the base A only
B) any of the three bases, U, C, and A
C) either U or A
D) any of the four bases, U, C, G, and A

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
7
The modified base 2-thiouracil found in tRNA can pair with
A) the base A only
B) any of the three bases, U, C, and A
C) either A or G
D) any of the four bases, U, C, G, and A
A) the base A only
B) any of the three bases, U, C, and A
C) either A or G
D) any of the four bases, U, C, G, and A

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
8
The "wobble hypothesis" states that
A) alternative (non-Watson-Crick) base-pairing is permitted between a codon and anticodon at the third position.
B) a peptide backbone can bend between the central carbon and the amino group's N or the carboxyl group's C.
C) an allosteric regulator can induce a protein to "wobble" between two conformations.
D) amino acids can spontaneously isomerize between the D- and L- forms.
A) alternative (non-Watson-Crick) base-pairing is permitted between a codon and anticodon at the third position.
B) a peptide backbone can bend between the central carbon and the amino group's N or the carboxyl group's C.
C) an allosteric regulator can induce a protein to "wobble" between two conformations.
D) amino acids can spontaneously isomerize between the D- and L- forms.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
9
The frequencies with which different codons are used vary significantly between different organisms and between proteins expressed at high or low levels within the same organism. This is referred to as:
A) stratification
B) codon variegation
C) heterologous codons
D) codon bias
A) stratification
B) codon variegation
C) heterologous codons
D) codon bias

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
10
At the pH found in cells (about 7.0), what happens to the amino group on an amino acid?
A) It acts as a base and gains a proton, giving it a positive charge.
B) It acts as a base and gains a proton, giving it a negative charge.
C) It acts as an acid and loses a proton, giving it a negative charge.
D) It neither gains nor loses a proton and does not have a charge.
A) It acts as a base and gains a proton, giving it a positive charge.
B) It acts as a base and gains a proton, giving it a negative charge.
C) It acts as an acid and loses a proton, giving it a negative charge.
D) It neither gains nor loses a proton and does not have a charge.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
11
At the pH found in cells (about 7.0), what happens to the carboxyl group on an amino acid?
A) It acts as a base and gains a proton, giving it a positive charge.
B) It acts as an acid and loses a proton, giving it a positive charge.
C) It acts as an acid and loses a proton, giving it a negative charge.
D) It neither gains nor loses a proton and does not have a charge.
A) It acts as a base and gains a proton, giving it a positive charge.
B) It acts as an acid and loses a proton, giving it a positive charge.
C) It acts as an acid and loses a proton, giving it a negative charge.
D) It neither gains nor loses a proton and does not have a charge.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
12
Suppose you have discovered a new amino acid. Its R-group contains only carbon and hydrogen atoms. Predict the behavior of this amino acid.
A) It is hydrophilic.
B) It is hydrophobic.
C) It is polar.
D) Its R-group is positively charged at physiological pH.
A) It is hydrophilic.
B) It is hydrophobic.
C) It is polar.
D) Its R-group is positively charged at physiological pH.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
13
Amino acids are joined by a condensation reaction to form a polypeptide chain with a ___________ backbone and ___________ side chain.
A) variable, common
B) variable, variable
C) common, variable
D) common, common
A) variable, common
B) variable, variable
C) common, variable
D) common, common

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
14
The peptide bond acts as a partial double bond as a result of
A) the acid-base properties of amino acids
B) wobble base pairing
C) the side chain or "R group"
D) resonance
A) the acid-base properties of amino acids
B) wobble base pairing
C) the side chain or "R group"
D) resonance

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
15
The primary structure of a protein is
A) the sequence of a chain of amino acids joined together by phosphodiester bonds.
B) the sequence of a chain of amino acids joined together by peptide bonds.
C) nearby amino acids in a chain joined together by hydrogen bonds.
D) the three-dimensional folding of amino acids in a chain.
A) the sequence of a chain of amino acids joined together by phosphodiester bonds.
B) the sequence of a chain of amino acids joined together by peptide bonds.
C) nearby amino acids in a chain joined together by hydrogen bonds.
D) the three-dimensional folding of amino acids in a chain.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
16
The C-terminus of a protein corresponds to
A) the R-group of the first amino acid
B) the R-group of the last amino acid
C) the amino group of the first amino acid
D) the carboxyl group of the last amino acid
A) the R-group of the first amino acid
B) the R-group of the last amino acid
C) the amino group of the first amino acid
D) the carboxyl group of the last amino acid

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
17
You have just sequenced a new protein found in Tetrahymena and observe that sulfur-containing cysteine residues occur at regular intervals in the sequence. Which hypothesis is supported by this observation?
A) This protein contains many alpha helices.
B) This protein contains many beta-pleated sheets.
C) This protein's tertiary structure is stabilized by disulfide bridges.
D) This protein's tertiary structure is stabilized by hydrogen bonding.
A) This protein contains many alpha helices.
B) This protein contains many beta-pleated sheets.
C) This protein's tertiary structure is stabilized by disulfide bridges.
D) This protein's tertiary structure is stabilized by hydrogen bonding.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
18
Which amino acid shown below, glutamine or valine, would be more likely to be found within the membrane region of a transmembrane protein, and less likely to be found in the intracellular region? 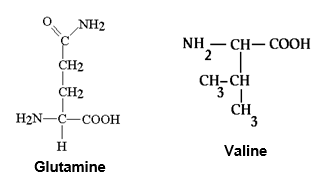
A) Glutamine
B) Valine

A) Glutamine
B) Valine

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
19
You have discovered an enzyme that has three active sites. When you denature the enzyme and study its composition, you find that each active site occurs on a different polypeptide. Which of the following hypotheses does this observation support?
A) The enzyme is subject to allosteric regulation.
B) The enzyme requires a cofactor to function normally.
C) The structure of the enzyme is affected by temperature and pH.
D) The enzyme has quaternary structure.
A) The enzyme is subject to allosteric regulation.
B) The enzyme requires a cofactor to function normally.
C) The structure of the enzyme is affected by temperature and pH.
D) The enzyme has quaternary structure.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
20
D- and L- amino acids
A) contain different R groups
B) are used in equal proportion in most proteins
C) are enantiomers
D) contain different numbers of backbone carbon atoms
A) contain different R groups
B) are used in equal proportion in most proteins
C) are enantiomers
D) contain different numbers of backbone carbon atoms

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
21
A particular polypeptide has a molecular weight of 40 kDa. The average molecular weight ofan amino acid is 110. The polypeptide chain thus contains approximately how many amino acids?
A) 3.6
B) 364
C) 4400
D) 4,400,000
A) 3.6
B) 364
C) 4400
D) 4,400,000

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
22
The Dalton unit, which is used to describe the mass of proteins, is defined as
A) one atomic mass unit.
B) the atomic mass of glycine, the simplest amino acid.
C) the average atomic mass of the 20 common amino acids.
D) the precise atomic mass of a given protein.
A) one atomic mass unit.
B) the atomic mass of glycine, the simplest amino acid.
C) the average atomic mass of the 20 common amino acids.
D) the precise atomic mass of a given protein.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
23
An average molecular weight for a protein is
A) 110 Daltons
B) 1100 Daltons (1.1 kiloDaltons)
C) 50,000 Daltons (50 kiloDaltons)
D) 500,000 Daltons (500 kiloDaltons)
A) 110 Daltons
B) 1100 Daltons (1.1 kiloDaltons)
C) 50,000 Daltons (50 kiloDaltons)
D) 500,000 Daltons (500 kiloDaltons)

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
24
Proline is a "helix-breaking" residue because
A) its R-group is too large to be accommodated in the alpha helix interior
B) its R-group is too hydrophobic to interact with water surrounding the alpha helix
C) its backbone nitrogen can only form hydrogen bonds in the context of a pleated beta sheet
D) cyclization prevents its backbone nitrogen from participating in hydrogen bonding that stabilizes the helix
A) its R-group is too large to be accommodated in the alpha helix interior
B) its R-group is too hydrophobic to interact with water surrounding the alpha helix
C) its backbone nitrogen can only form hydrogen bonds in the context of a pleated beta sheet
D) cyclization prevents its backbone nitrogen from participating in hydrogen bonding that stabilizes the helix

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
25
An alpha helix is stabilized by
A) van der Waals interactions between R-group atoms in the helix interior
B) ionic bonding between R-group atoms of residues located near one another
C) hydrogen bonding between backbone atoms of residues located near one another
D) peptide bonding between backbone atoms of non-adjacent residues
A) van der Waals interactions between R-group atoms in the helix interior
B) ionic bonding between R-group atoms of residues located near one another
C) hydrogen bonding between backbone atoms of residues located near one another
D) peptide bonding between backbone atoms of non-adjacent residues

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
26
A beta-pleated sheet is stabilized by
A) hydrogen bonding between backbone atoms of residues located in adjacent beta strands
B) van der Waals interactions between R-group atoms that extend perpendicularly from the plane of the sheet
C) ionic bonding between R-group atoms of residues located near one another
D) peptide bonding between backbone atoms of adjacent beta strands
A) hydrogen bonding between backbone atoms of residues located in adjacent beta strands
B) van der Waals interactions between R-group atoms that extend perpendicularly from the plane of the sheet
C) ionic bonding between R-group atoms of residues located near one another
D) peptide bonding between backbone atoms of adjacent beta strands

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
27
Short unstructured regions of a protein that connect secondary structural elements are called
A) beta hooks
B) turns
C) barrels
D) branch points
A) beta hooks
B) turns
C) barrels
D) branch points

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
28
In a beta-pleated sheet,
A) adjacent polypeptide strands interact in an extended conformation
B) beta strands stack at perpendicular angles
C) R-groups of hydrophobic amino acids stabilize adjacent stretches of amino acid residues
D) alpha helices align in parallel or antiparallel arrays
A) adjacent polypeptide strands interact in an extended conformation
B) beta strands stack at perpendicular angles
C) R-groups of hydrophobic amino acids stabilize adjacent stretches of amino acid residues
D) alpha helices align in parallel or antiparallel arrays

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is NOT true about protein structure?
A) A protein's tertiary structure is ultimately determined by its primary amino acid sequence.
B) An alpha helix is an example of tertiary structure.
C) Tertiary structure may be stabilized by hydrogen bonds, disulfide bonds, ionic bridges, and van der Waals interactions
D) Enzymes such as lysozyme have tertiary structure.
A) A protein's tertiary structure is ultimately determined by its primary amino acid sequence.
B) An alpha helix is an example of tertiary structure.
C) Tertiary structure may be stabilized by hydrogen bonds, disulfide bonds, ionic bridges, and van der Waals interactions
D) Enzymes such as lysozyme have tertiary structure.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
30
A common structural element in fibrous proteins is
A) transmembrane domain.
B) beta pleated sheet.
C) hydrophobic core.
D) coiled coil.
A) transmembrane domain.
B) beta pleated sheet.
C) hydrophobic core.
D) coiled coil.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
31
You have isolated a novel protein, characterized its structure and determined that it catalyzes the breakdown of a large substrate and has two binding sites. One of these is large, apparently the binding site for the large substrate; the other is small, possibly a binding site for a regulatory molecule. What do these findings suggest to you about the mode of action of this protein?
A) It is probably a structural protein that is found in tendons or bone.
B) It is probably an enzyme that is controlled through allosteric regulation.
C) It is probably an enzyme that works through autophosphorylation.
D) It is probably a transmembrane protein-like a G protein-coupled receptor.
A) It is probably a structural protein that is found in tendons or bone.
B) It is probably an enzyme that is controlled through allosteric regulation.
C) It is probably an enzyme that works through autophosphorylation.
D) It is probably a transmembrane protein-like a G protein-coupled receptor.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following illustrates quaternary protein structure?
A) an allosteric ligand
B) an actin monomer
C) hemoglobin
D) a metal-binding protein
A) an allosteric ligand
B) an actin monomer
C) hemoglobin
D) a metal-binding protein

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
33
Which level(s) of protein structure could be disrupted by treatment with a reducing agent?
A) primary
B) primary and secondary
C) tertiary
D) tertiary and quaternary
A) primary
B) primary and secondary
C) tertiary
D) tertiary and quaternary

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
34
An enzyme's active site usually
A) is on the outer surface of an enzyme.
B) is hydrophobic.
C) forms a cleft or pocket in the enzyme.
D) undergoes irreversible modification during catalysis.
A) is on the outer surface of an enzyme.
B) is hydrophobic.
C) forms a cleft or pocket in the enzyme.
D) undergoes irreversible modification during catalysis.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
35
The term induced fit refers to
A) the coalescence of nonpolar amino acid residues in a protein's amino acid sequence into a hydrophobic core.
B) a conformational change in an enzyme upon substrate binding.
C) the process of inducing a protein to fold into its lowest energy tertiary structure.
D) any specific protein-protein interaction.
A) the coalescence of nonpolar amino acid residues in a protein's amino acid sequence into a hydrophobic core.
B) a conformational change in an enzyme upon substrate binding.
C) the process of inducing a protein to fold into its lowest energy tertiary structure.
D) any specific protein-protein interaction.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
36
Extrapolating from the example of lysozyme, which part of an enzyme do you suppose directly promotes catalysis?
A) the peptide backbone
B) amino acid side chains
C) alpha helices
D) the hydrophobic core
A) the peptide backbone
B) amino acid side chains
C) alpha helices
D) the hydrophobic core

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
37
An enzyme that catalyzes phosphorylation of a substrate is called
A) a kinase.
B) a transferase.
C) a phosphoprotein.
D) a glycoprotein.
A) a kinase.
B) a transferase.
C) a phosphoprotein.
D) a glycoprotein.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
38
Many steps in gene expression and cell signaling involve post-translational modification of proteins by
A) reversible disulfide bridge formation.
B) irreversible disulfide bridge formation.
C) reversible phosphorylation.
D) irreversible phosphorylation.
A) reversible disulfide bridge formation.
B) irreversible disulfide bridge formation.
C) reversible phosphorylation.
D) irreversible phosphorylation.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
39
Cyclin-dependent kinase activity is regulated by
A) phosphorylation.
B) allosteric modification.
C) allolactose.
D) both A and B
A) phosphorylation.
B) allosteric modification.
C) allolactose.
D) both A and B

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
40
Allosteric regulation always involves
A) a change in protein shape or conformation.
B) a post-translational modification.
C) interaction with Hsp90.
D) the ubiquitin-proteasome system.
A) a change in protein shape or conformation.
B) a post-translational modification.
C) interaction with Hsp90.
D) the ubiquitin-proteasome system.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is not true about molecular chaperones?
A) They decrease the efficiency of the overall process of protein folding.
B) Some of them enhance the rate of formation of disulfide bonds.
C) They reduce the probability of protein aggregation.
D) They aid in the destruction of misfolded proteins.
A) They decrease the efficiency of the overall process of protein folding.
B) Some of them enhance the rate of formation of disulfide bonds.
C) They reduce the probability of protein aggregation.
D) They aid in the destruction of misfolded proteins.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
42
You determine that a protein has an attached polyubiquitin chain. It is likely that this protein will be
A) targeted to the lysosome for degradation.
B) targeted to the 26S proteosome for degradation.
C) included in insoluble toxic deposits known as amyloids fibers.
D) degraded by E1, E2, and E3 enzymes.
A) targeted to the lysosome for degradation.
B) targeted to the 26S proteosome for degradation.
C) included in insoluble toxic deposits known as amyloids fibers.
D) degraded by E1, E2, and E3 enzymes.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
43
Alzheimer's disease, Huntington's disease, and Creutzfeld-Jakob disease are all examples of:
A) transmissible spongiform encephalopathies.
B) trinucleotide repeat disorders.
C) protein misfolding diseases.
D) diseases that are not related in any way.
A) transmissible spongiform encephalopathies.
B) trinucleotide repeat disorders.
C) protein misfolding diseases.
D) diseases that are not related in any way.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
44
A prion is an infectious protein
A) with an excess of phosphate modifications.
B) associated with viruses.
C) that induces genetic changes (changes in DNA sequence).
D) with an altered conformation.
A) with an excess of phosphate modifications.
B) associated with viruses.
C) that induces genetic changes (changes in DNA sequence).
D) with an altered conformation.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
45
Do UAG, UAA, and UGA always act as termination codons during protein synthesis? Explain your answer.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
46
You are attempting to express a 50 kDa Tetrahymena protein from a cloned gene introduced into E. coli. When you purify the overexpressed protein you find that it is only 25 kDa in size. Discuss possible reasons for this result.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
47
Draw a clearly-labeled diagram showing where the linking bond is between two amino acids in a polypeptide chain. Provide the name of the bond.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
48
Use a rough diagram to compare the structures of a protein alpha-helix and a beta-pleated sheet. For simplicity, show only the backbone of the polypeptide chain.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
49
What is meant by primary, secondary, tertiary, and quaternary structures of proteins?

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
50
Compare and contrast the overall shape and function of globular, fibrous, and membrane proteins. Give a specific example of a protein with each type of tertiary structure.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
51
You have determined the amino acid sequence of a new protein. Is it possible to determine the three-dimensional structure of the protein from its sequence? Why or why not?

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
52
Draw a graph comparing progress of a reaction with or without a catalyst. Label the axes, EA, and

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
53
Explain the "induced-fit" model for enzyme action.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
54
What does a kinase do?

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
55
Describe a specific example of control of enzyme activity by post-translational modification and allosteric regulation.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
56
Discuss the roles of molecular chaperones in protein folding and degradation.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
57
Describe two possible fates for a misfolded protein in a cell.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
58
You treat cells with an inhibitor of the 26S proteasome. Predict the effect on cellular protein levels.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
59
Use a diagram to outline the key steps in ubiquitin-mediated protein degradation.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
60
The sequence of a portion of a gene is presented below:
5′-AGCAATGCATGCATCGTTATGG-3′
3′-TCGTTACGTACGTAGCAATACC-5′
(a) Assuming that transcription starts with the first T in the template strand of the DNA, and continues to the end, what would be the sequence of the transcribed mRNA?
(b) Identify the initiation codon in this mRNA.
(c) Would there be an effect on translation of changing the first C in the template strand to a G? If so, what effect?
(d) Would there be an effect on translation of changing the third G in the template strand to a T? If so, what effect?
(e) Would there be an effect on translation of changing the last A in the template strand to a C? If so, what effect?
(f) Would there be an effect on translation if pyrrolysine were present?
5′-AGCAATGCATGCATCGTTATGG-3′
3′-TCGTTACGTACGTAGCAATACC-5′
(a) Assuming that transcription starts with the first T in the template strand of the DNA, and continues to the end, what would be the sequence of the transcribed mRNA?
(b) Identify the initiation codon in this mRNA.
(c) Would there be an effect on translation of changing the first C in the template strand to a G? If so, what effect?
(d) Would there be an effect on translation of changing the third G in the template strand to a T? If so, what effect?
(e) Would there be an effect on translation of changing the last A in the template strand to a C? If so, what effect?
(f) Would there be an effect on translation if pyrrolysine were present?

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
61
Replacing an A with a T in a hemoglobin gene is associated with sickle-cell anemia in humans.
Normal: 5′-ATGGTGCACCTGACTCCTGAGGAGAAGTCT-3′
Sickle cell: 5′-ATGGTGCACCTGACTCCTGTGGAGAAGTCT-3′
(a) What is the nucleotide sequence of the normal and sickle-cell hemoglobin mRNA?
(b) What is the amino acid sequence in this part of the polypeptide chain, and what is the amino acid replacement that results in sickle-cell hemoglobin?
(c) Why might this amino acid substitution make a difference in protein structure?
Normal: 5′-ATGGTGCACCTGACTCCTGAGGAGAAGTCT-3′
Sickle cell: 5′-ATGGTGCACCTGACTCCTGTGGAGAAGTCT-3′
(a) What is the nucleotide sequence of the normal and sickle-cell hemoglobin mRNA?
(b) What is the amino acid sequence in this part of the polypeptide chain, and what is the amino acid replacement that results in sickle-cell hemoglobin?
(c) Why might this amino acid substitution make a difference in protein structure?

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
62
You determine the structure of a protein of unknown function. The protein adopts a filamentous coiled-coil of two alpha-helices. Is the protein likely to be an enzyme? Explain.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
63
Provide an explanation for why you should politely decline a serving of brains at a party hosted by cannibals.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck


