Deck 11: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/125
Play
Full screen (f)
Deck 11: Acids and Bases
1
Which of the following compounds is not an Arrhenius acid?
A) HCl
B) H2SO4
C) H2O
D) FeCl3
E) H2S
A) HCl
B) H2SO4
C) H2O
D) FeCl3
E) H2S
FeCl3
2
Which of the following compounds is not an Arrhenius base?
A) NaOH
B) Al(OH)3
C) Ca(OH)2
D) H2O
E) NH3
A) NaOH
B) Al(OH)3
C) Ca(OH)2
D) H2O
E) NH3
NH3
3
Consider the following reaction:
H2PO4-(aq) + HCO3-(aq)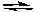 H2CO3(aq) + HPO42-(aq)
H2CO3(aq) + HPO42-(aq)
Brønsted would identify the acidic species as:
A) H2PO4- and H2CO3
B) H2PO4- and HPO42-
C) HCO3- and H2CO3
D) HCO3- and HPO42-
E) H2CO3 and HPO42-
H2PO4-(aq) + HCO3-(aq)
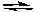 H2CO3(aq) + HPO42-(aq)
H2CO3(aq) + HPO42-(aq)Brønsted would identify the acidic species as:
A) H2PO4- and H2CO3
B) H2PO4- and HPO42-
C) HCO3- and H2CO3
D) HCO3- and HPO42-
E) H2CO3 and HPO42-
H2PO4- and H2CO3
4
Which compound cannot act as a Brønsted base?
A) NH3
B) H2O
C) CH4
D) F-
E) H2S
A) NH3
B) H2O
C) CH4
D) F-
E) H2S

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds cannot be a base?
A) H2O
B) NH3
C) CO32-
D) OH-
E) NH4+
A) H2O
B) NH3
C) CO32-
D) OH-
E) NH4+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following isn't a Brønsted base?
A) PF5
B) H2O
C) NH3
D) S2-
E) CN-
A) PF5
B) H2O
C) NH3
D) S2-
E) CN-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
7
What is the conjugate acid of hydrogen carbonate ion, HCO3 - ?
A) H3O+
B) OH -
C) H2O
D) CO32 -
E) H2CO3
A) H3O+
B) OH -
C) H2O
D) CO32 -
E) H2CO3

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
8
What is the conjugate base of thiocyanic acid, HSCN?
A) H3O+
B) OH -
C) SCN -
D) CN -
E) H2SCN+
A) H3O+
B) OH -
C) SCN -
D) CN -
E) H2SCN+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
9
The dihydrogen phosphate ion, H2PO4 - , can behave as both an acid and as a base. What is the chemical formula of the conjugate acid of dihydrogen phosphate? What is the chemical formula of the conjugate base of dihydrogen phosphate?
A) conjugate acid = H3PO4 conjugate base = HPO42 -
B) conjugate acid = H2PO4+ conjugate base = H2PO42 -
C) conjugate acid = HPO42 - conjugate base = H3PO4
D) conjugate acid = H3PO4 conjugate base = PO43 -
E) none of these
A) conjugate acid = H3PO4 conjugate base = HPO42 -
B) conjugate acid = H2PO4+ conjugate base = H2PO42 -
C) conjugate acid = HPO42 - conjugate base = H3PO4
D) conjugate acid = H3PO4 conjugate base = PO43 -
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
10
Ethanol (CH3CH2OH), like water, can behave both as an acid or as a base. Which of the following structures corresponds to the Brønsted conjugate acid and conjugate base of ethanol?

A)
B)
C)
D)
E) none of these

A)

B)

C)

D)

E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
11
What is the conjugate base of HSO4-?
A) H2O
B) H2SO4
C) HSO4-
D) SO3
E) none of the above
A) H2O
B) H2SO4
C) HSO4-
D) SO3
E) none of the above

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
12
Methanol is described by the following skeleton structure.
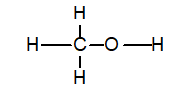
Which substance is formed when CH3OH acts as a Brønsted base?
A) CH3O-
B) CH3OH
C) CH3OH2+
D) CH3OH.
E) H3O+

Which substance is formed when CH3OH acts as a Brønsted base?
A) CH3O-
B) CH3OH
C) CH3OH2+
D) CH3OH.
E) H3O+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
13
The compound HB can be represented as 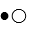 where
where 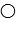 represent B- and
represent B- and  represents H+. Given this, ___ would represent the conjugate acid of HB and ____ would represent the conjugate base.
represents H+. Given this, ___ would represent the conjugate acid of HB and ____ would represent the conjugate base.
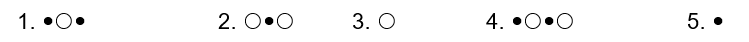
A) 1,2
B) 1,5
C) 3,4
D) 1,3
E) None of the above.
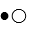 where
where 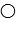 represent B- and
represent B- and  represents H+. Given this, ___ would represent the conjugate acid of HB and ____ would represent the conjugate base.
represents H+. Given this, ___ would represent the conjugate acid of HB and ____ would represent the conjugate base.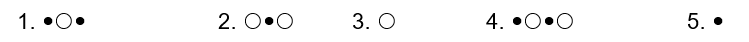
A) 1,2
B) 1,5
C) 3,4
D) 1,3
E) None of the above.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is the conjugate base of a strong acid?
A) OH-
B) HSO4-
C) NH2-
D) S2-
E) H3O+
A) OH-
B) HSO4-
C) NH2-
D) S2-
E) H3O+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following represents a conjugate acid/base pair?
A) H+, OH-
B) H3O+, OH-
C) NH3, NH4+
D) NaOH, HCl
E) All of the above are conjugate acid/base pairs.
A) H+, OH-
B) H3O+, OH-
C) NH3, NH4+
D) NaOH, HCl
E) All of the above are conjugate acid/base pairs.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
16
A solution is known to have a hydronium ion concentration of 1.0 x 10-6 M. What percentage of the total H3O+ ion concentration comes from the dissociation of water?
A) 1%
B) 3%
C) 5%
D) 10%
E) 100%
A) 1%
B) 3%
C) 5%
D) 10%
E) 100%

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
17
What is the [H3O+] concentration of a solution with a pH of 8.75?
A) 1.8 x 10 - 9M
B) 9.4 x 10 - 1M
C) 8.7 x 10-5M
D) 5.6 x 108M
E) This is a basic solution, [H3O+] cannot be determined.
A) 1.8 x 10 - 9M
B) 9.4 x 10 - 1M
C) 8.7 x 10-5M
D) 5.6 x 108M
E) This is a basic solution, [H3O+] cannot be determined.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
18
A solution of weak base is added to a beaker of water. Which of the following will be true as the base is added to the water?
A) [H3O+] increases [OH - ] decreases pH increases
B) [H3O+] decreases [OH -F ] increases pH decreases
C) [H3O+] decreases [OH - ] decreases pH increases
D) [H3O+] remains constant [OH - ] increases pH increases
E) none of these
A) [H3O+] increases [OH - ] decreases pH increases
B) [H3O+] decreases [OH -F ] increases pH decreases
C) [H3O+] decreases [OH - ] decreases pH increases
D) [H3O+] remains constant [OH - ] increases pH increases
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
19
Determine the [OH ] for an aqueous solution at 25 ![<strong>Determine the [OH <sup></sup> ] for an aqueous solution at 25 C with a pH of 4.80.</strong> A) 6.3x10 <sup></sup> <sup>10</sup>M B) 1.6x10 <sup></sup> <sup>5</sup>M C) 1.6x10 <sup></sup> <sup>9</sup>M D) 8.0x10 <sup></sup> <sup>8</sup>M E) none of these](https://storage.examlex.com/TB9692/11ee9f01_38f8_89d9_9fac_1f2262b4b888_TB9692_11.jpg) C with a pH of 4.80.
C with a pH of 4.80.
A) 6.3x10 10M
B) 1.6x10 5M
C) 1.6x10 9M
D) 8.0x10 8M
E) none of these
![<strong>Determine the [OH <sup></sup> ] for an aqueous solution at 25 C with a pH of 4.80.</strong> A) 6.3x10 <sup></sup> <sup>10</sup>M B) 1.6x10 <sup></sup> <sup>5</sup>M C) 1.6x10 <sup></sup> <sup>9</sup>M D) 8.0x10 <sup></sup> <sup>8</sup>M E) none of these](https://storage.examlex.com/TB9692/11ee9f01_38f8_89d9_9fac_1f2262b4b888_TB9692_11.jpg) C with a pH of 4.80.
C with a pH of 4.80.A) 6.3x10 10M
B) 1.6x10 5M
C) 1.6x10 9M
D) 8.0x10 8M
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
20
A strong acid is added to water. Which of the following will be true?
A) [H3O+] increases, [OH - ] decreases, pH increases
B) [H3O+] decreases, [OH - ] increases, pH decreases
C) [H3O+] decreases, [OH - ] decreases, pH decreases
D) [H3O+] increases, [OH - ] increases, pH increases
E) none of these
A) [H3O+] increases, [OH - ] decreases, pH increases
B) [H3O+] decreases, [OH - ] increases, pH decreases
C) [H3O+] decreases, [OH - ] decreases, pH decreases
D) [H3O+] increases, [OH - ] increases, pH increases
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
21
What is the H3O+ ion concentration in a solution that has a pH of 5.75?
A) 5.8 x 10-5 M
B) 3.2 x 10-3 M
C) 0.75 x 10-5 M
D) 3.6 x 10-7 M
E) 1.8 x 10-6 M
A) 5.8 x 10-5 M
B) 3.2 x 10-3 M
C) 0.75 x 10-5 M
D) 3.6 x 10-7 M
E) 1.8 x 10-6 M

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
22
What is the pH of a solution that is 1.7 x 10-4 M in H3O+?
A) 3.77
B) 4.77
C) 4.23
D) 10.23
E) none of these
A) 3.77
B) 4.77
C) 4.23
D) 10.23
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
23
What is the hydroxide ion concentration in a pH = 5.14 solution?
A) 1.0 x 10-14
B) 1.4 x 10-9
C) 1.0 x 10-7
D) 7.2 x 10-6
E) 1.4 x 10-2
A) 1.0 x 10-14
B) 1.4 x 10-9
C) 1.0 x 10-7
D) 7.2 x 10-6
E) 1.4 x 10-2

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
24
Which equation best describes a 0.10 M solution of a strong acid such as hydroiodic acid (HI: Ka = 3 x 109)?
A) [H3O+] = initial concentration of HI
B) [H3O+] [OH-]
C) [H3O+] final concentration of HI
D) [H3O+] [H2O]
E) [H3O+] Ka.[OH-]
A) [H3O+] = initial concentration of HI
B) [H3O+] [OH-]
C) [H3O+] final concentration of HI
D) [H3O+] [H2O]
E) [H3O+] Ka.[OH-]

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following acids would have the strongest conjugate base?
A) HOCl (Ka = 2.9 x 10-8)
B) HOBr (Ka = 2.4 x 10-9)
C) HOI (Ka = 2.3 x 10-11)
D) H2O2 (Ka = 2.2 x 10-12)
E) H2O (Ka = 1.8 x 10-16)
A) HOCl (Ka = 2.9 x 10-8)
B) HOBr (Ka = 2.4 x 10-9)
C) HOI (Ka = 2.3 x 10-11)
D) H2O2 (Ka = 2.2 x 10-12)
E) H2O (Ka = 1.8 x 10-16)

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
26
Using a table of Ka's, determine which of the following is the strongest Brønsted acid.
A) NH4+
B) HCl
C) HNO3
D) HSO4-
E) HClO4
A) NH4+
B) HCl
C) HNO3
D) HSO4-
E) HClO4

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
27
Using a table of Ka's, determine which of the following is the strongest base.
A) I-
B) NO3-
C) HS-
D) O2-
E) OH-
A) I-
B) NO3-
C) HS-
D) O2-
E) OH-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
28
Using a table of Ka's, determine which of the following is the strongest acid.
A) NH2-
B) HS-
C) H2O
D) CH4
E) NH4+
A) NH2-
B) HS-
C) H2O
D) CH4
E) NH4+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
29
If the strength of the following acids increases from left to right
CH3NH2 < CH3NH3+ < C6H5NH3 + < C6H5CO2H < HCl
The order of increasing base strength, reading from left to right, must be:
A) CH3NH- < CH3NH2 < C6H5NH2 < C6H5CO2- < Cl-
B) Cl- < C6H5CO2- < C6H5NH2 < CH3NH2 < CH3NH-
C) C6H5NH2 < CH3NH2 < Cl- < C6H5CO2- < CH3NH-
D) CH3NH- < C6H5CO2- < Cl- < CH3NH2 < C6H5NH2
E) none of the above
CH3NH2 < CH3NH3+ < C6H5NH3 + < C6H5CO2H < HCl
The order of increasing base strength, reading from left to right, must be:
A) CH3NH- < CH3NH2 < C6H5NH2 < C6H5CO2- < Cl-
B) Cl- < C6H5CO2- < C6H5NH2 < CH3NH2 < CH3NH-
C) C6H5NH2 < CH3NH2 < Cl- < C6H5CO2- < CH3NH-
D) CH3NH- < C6H5CO2- < Cl- < CH3NH2 < C6H5NH2
E) none of the above

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
30
Explain the following observation: Water becomes acidic when CO2 is bubbled into it.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is the weakest acid?
A) HClO4
B) HCl
C) HF
D) HI
E) HBr
A) HClO4
B) HCl
C) HF
D) HI
E) HBr

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
32
Arrange the following bases in order of increasing strength: NH3, PH3, H2O and H2S.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements is true?
A) H2O is a stronger acid than HF(aq).
B) H2O is a stronger acid than H2S(aq).
C) H2O is a stronger acid than the H3O+(aq) ion.
D) H2O is a stronger acid than NH3(aq).
E) None of the above are true.
A) H2O is a stronger acid than HF(aq).
B) H2O is a stronger acid than H2S(aq).
C) H2O is a stronger acid than the H3O+(aq) ion.
D) H2O is a stronger acid than NH3(aq).
E) None of the above are true.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
34
What can be correctly concluded from the fact that the following acid-base reaction proceeds to the right, as written?
NH2-(aq) + HSO4-(aq) NH3(aq) + SO42-(aq)
NH3(aq) + SO42-(aq)
A) NH3 is a stronger base than NH2-.
B) NH3 is a stronger acid than HSO4-.
C) NH3 is a weaker acid than NH2-.
D) NH3 is a weaker base than HSO4-.
E) NH3 is a weaker acid than HSO4-.
NH2-(aq) + HSO4-(aq)
 NH3(aq) + SO42-(aq)
NH3(aq) + SO42-(aq)A) NH3 is a stronger base than NH2-.
B) NH3 is a stronger acid than HSO4-.
C) NH3 is a weaker acid than NH2-.
D) NH3 is a weaker base than HSO4-.
E) NH3 is a weaker acid than HSO4-.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements is true?
A) H2O is a weaker acid than NH3.
B) PH3 is a weaker acid than NH3.
C) OH- is a weaker base than H2O.
D) NH2- is the conjugate acid of NH3.
E) ClO- is a stronger base than ClO4-.
A) H2O is a weaker acid than NH3.
B) PH3 is a weaker acid than NH3.
C) OH- is a weaker base than H2O.
D) NH2- is the conjugate acid of NH3.
E) ClO- is a stronger base than ClO4-.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
36
The hydride ion, (H-), is a stronger base than the hydroxide ion, (OH-). The products of the reaction between H- (aq) and H2O(l) would be.
A) H2O(aq)
B) OH- (aq) + H2(g)
C) OH- (aq) + 2H+(aq)
D) H2O2(aq)
E) No reaction would occur.
A) H2O(aq)
B) OH- (aq) + H2(g)
C) OH- (aq) + 2H+(aq)
D) H2O2(aq)
E) No reaction would occur.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following structural factors is primarily responsible for H2Se being a stronger acid than H2S?
A) bond polarity
B) size of the anion
C) electron withdrawing effect of oxygen
D) electric charge on the acid
E) sulfur has d electrons
A) bond polarity
B) size of the anion
C) electron withdrawing effect of oxygen
D) electric charge on the acid
E) sulfur has d electrons

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is the best Lewis structure for carbonic acid (H2CO3)?
A)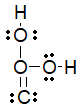
B)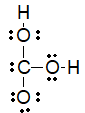
C)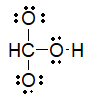
D)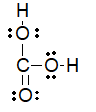
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
39
Of the following, which is the strongest acid (lone pairs omitted for clarity)?
A)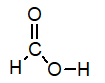
B)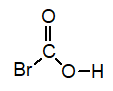
C)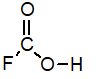
D)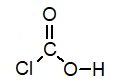
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following structural factors is primarily responsible for H2AsO4 - being a stronger acid than HAsO42 - ?
A) bond polarity
B) size of the anion
C) electron withdrawing effect of oxygen
D) electric charge on the acid
E) arsenic has d electrons
A) bond polarity
B) size of the anion
C) electron withdrawing effect of oxygen
D) electric charge on the acid
E) arsenic has d electrons

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following would you expect to be the strongest acid?
A) NH3
B) PH3
C) H2O
D) H2S
E) CH4
A) NH3
B) PH3
C) H2O
D) H2S
E) CH4

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following would be the weakest Brønsted acid?
A) H2Se
B) H2S
C) H2O
D) SH-
E) OH-
A) H2Se
B) H2S
C) H2O
D) SH-
E) OH-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following would be the strongest Brønsted base?
A) HSO3-
B) HSO4-
C) H2SO3
D) SO32-
E) SO42-
A) HSO3-
B) HSO4-
C) H2SO3
D) SO32-
E) SO42-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following would be the strongest Brønsted base?
A) H2O
B) SH-
C) OH-
D) S2-
E) O2-
A) H2O
B) SH-
C) OH-
D) S2-
E) O2-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
45
What is the pOH of a 0.025M solution of hydrobromic acid (Ka = 1 x 109)?
A) 0.025
B) 1.60
C) 12.40
D) 9.00
E) 5.00
A) 0.025
B) 1.60
C) 12.40
D) 9.00
E) 5.00

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
46
What is the pH of a 0.0250 M HClO4 solution.
A) 2.50
B) -2.50
C) 1.60
D) 12.40
E) none of these
A) 2.50
B) -2.50
C) 1.60
D) 12.40
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following statements should be true for a 1.0x 10 - 4 M solution of a strong acid HBr?
A) [Br - ] >> [H3O+]
B) pH = 4.00
C) [HBr] = 1.0 x 10 - 4M
D) [Br - ] = [HBr]
E) pH = 1.00
A) [Br - ] >> [H3O+]
B) pH = 4.00
C) [HBr] = 1.0 x 10 - 4M
D) [Br - ] = [HBr]
E) pH = 1.00

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements is true for a 1.0 M solution of the strong acid HCl in water?
A) [Cl - ] > [H3O+]
B) The pH is 1.00
C) [H3O+] = 1.0 M
D) [HCl] = 1.0 M
E) [H3O+] = [OH - ]
A) [Cl - ] > [H3O+]
B) The pH is 1.00
C) [H3O+] = 1.0 M
D) [HCl] = 1.0 M
E) [H3O+] = [OH - ]

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
49
Assume a solution is prepared by adding 1.0 x 10-3 moles of a weak monoprotic acid (Ka = 2.0 x 10-4) to enough water to give a liter of solution. If you want to compute the H3O+ ion concentration with an error of 5% or less, you can legitimately ignore:
A) both the contribution of the dissociation of water and the C term.
B) neither the dissociation of water nor the C term.
C) the dissociation of water but not the C term.
D) the C term but not the dissociation of water.
E) You can't compute the H3O+ concentration with an error of less than 5%.
A) both the contribution of the dissociation of water and the C term.
B) neither the dissociation of water nor the C term.
C) the dissociation of water but not the C term.
D) the C term but not the dissociation of water.
E) You can't compute the H3O+ concentration with an error of less than 5%.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
50
When 1.0 x 10-5 mole of HOCl (Ka = 3.5 x 10-8) is dissolved in pure water and diluted to 1.00 L, which assumption can't be applied in the calculation of the pH of this solution?
A) that the initial concentration of HOCl is much larger than the total H3O+ ion concentration
B) that the H3O+ ion concentration from the dissociation of water can be ignored.
C) that the total H3O+ ion concentration is the sum of the concentrations from the dissociation of HOCl and water
D) all of these assumptions are valid
E) none of these assumptions are valid
A) that the initial concentration of HOCl is much larger than the total H3O+ ion concentration
B) that the H3O+ ion concentration from the dissociation of water can be ignored.
C) that the total H3O+ ion concentration is the sum of the concentrations from the dissociation of HOCl and water
D) all of these assumptions are valid
E) none of these assumptions are valid

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
51
For a particular weak acid, HA, in pure water, as the initial concentration of the weak acid increases, the acid-dissociation equilibrium constant of that acid should
A) increase.
B) decrease.
C) remain the same.
D) increase until the ionization of water can be neglected.
E) decrease until the ionization of water can be neglected.
A) increase.
B) decrease.
C) remain the same.
D) increase until the ionization of water can be neglected.
E) decrease until the ionization of water can be neglected.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
52
What would happen if more formic acid (HCO2H) was added to the following solution at equilibrium?
HCO2H(aq) + H2O(l)![<strong>What would happen if more formic acid (HCO<sub>2</sub>H) was added to the following solution at equilibrium? HCO<sub>2</sub>H(aq) + H<sub>2</sub>O(l) H<sub>3</sub>O<sup>+</sup>(aq) + HCO<sub>2</sub><sup>-</sup>(aq)</strong> A) [H<sub>2</sub>O] should increase. B) [H<sub>3</sub>O<sup>+</sup>] and [HCO<sub>2</sub><sup>-</sup>] should both increase. C) [H<sub>3</sub>O<sup>+</sup>] and [HCO<sub>2</sub><sup>-</sup>] should both decrease. D) [H<sub>3</sub>O<sup>+</sup>] should increase but [HCO<sub>2</sub><sup>-</sup>] should decrease. E) [H<sub>3</sub>O<sup>+</sup>] should decrease but [HCO<sub>2</sub><sup>-</sup>] should increase.](https://storage.examlex.com/TB9692/11ee9da3_9df0_ade8_9ff6_e3e6f1ad2e34_TB9692_11.jpg) H3O+(aq) + HCO2-(aq)
H3O+(aq) + HCO2-(aq)
A) [H2O] should increase.
B) [H3O+] and [HCO2-] should both increase.
C) [H3O+] and [HCO2-] should both decrease.
D) [H3O+] should increase but [HCO2-] should decrease.
E) [H3O+] should decrease but [HCO2-] should increase.
HCO2H(aq) + H2O(l)
![<strong>What would happen if more formic acid (HCO<sub>2</sub>H) was added to the following solution at equilibrium? HCO<sub>2</sub>H(aq) + H<sub>2</sub>O(l) H<sub>3</sub>O<sup>+</sup>(aq) + HCO<sub>2</sub><sup>-</sup>(aq)</strong> A) [H<sub>2</sub>O] should increase. B) [H<sub>3</sub>O<sup>+</sup>] and [HCO<sub>2</sub><sup>-</sup>] should both increase. C) [H<sub>3</sub>O<sup>+</sup>] and [HCO<sub>2</sub><sup>-</sup>] should both decrease. D) [H<sub>3</sub>O<sup>+</sup>] should increase but [HCO<sub>2</sub><sup>-</sup>] should decrease. E) [H<sub>3</sub>O<sup>+</sup>] should decrease but [HCO<sub>2</sub><sup>-</sup>] should increase.](https://storage.examlex.com/TB9692/11ee9da3_9df0_ade8_9ff6_e3e6f1ad2e34_TB9692_11.jpg) H3O+(aq) + HCO2-(aq)
H3O+(aq) + HCO2-(aq)A) [H2O] should increase.
B) [H3O+] and [HCO2-] should both increase.
C) [H3O+] and [HCO2-] should both decrease.
D) [H3O+] should increase but [HCO2-] should decrease.
E) [H3O+] should decrease but [HCO2-] should increase.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following equations is valid for a 0.10 M solution of formic acid, HCO2H?
HCO2H(aq) + H2O(l)![<strong>Which of the following equations is valid for a 0.10 M solution of formic acid, HCO<sub>2</sub>H? HCO<sub>2</sub>H(aq) + H<sub>2</sub>O(l) HCO<sub>2</sub><sup>-</sup>(aq) + H<sub>3</sub>O<sup>+</sup>(aq) K<sub>a</sub> = 1.8 x 10<sup>-4</sup></strong> A) [H<sub>3</sub>O<sup>+</sup>] < [OH<sup>-</sup>] B) [H<sub>3</sub>O<sup>+</sup>]<sub> </sub>= [HCO<sub>2</sub>H] C) [H<sub>3</sub>O<sup>+</sup>]<sub> </sub>= [HCO<sub>2</sub><sup>-</sup>] D) [H<sub>3</sub>O<sup>+</sup>] = [H<sub>2</sub>O] E) [OH<sup>-</sup>] = [H<sub>2</sub>O]](https://storage.examlex.com/TB9692/11ee9da3_9df0_ade9_9ff6_9f249b101645_TB9692_11.jpg) HCO2-(aq) + H3O+(aq) Ka = 1.8 x 10-4
HCO2-(aq) + H3O+(aq) Ka = 1.8 x 10-4
A) [H3O+] < [OH-]
B) [H3O+] = [HCO2H]
C) [H3O+] = [HCO2-]
D) [H3O+] = [H2O]
E) [OH-] = [H2O]
HCO2H(aq) + H2O(l)
![<strong>Which of the following equations is valid for a 0.10 M solution of formic acid, HCO<sub>2</sub>H? HCO<sub>2</sub>H(aq) + H<sub>2</sub>O(l) HCO<sub>2</sub><sup>-</sup>(aq) + H<sub>3</sub>O<sup>+</sup>(aq) K<sub>a</sub> = 1.8 x 10<sup>-4</sup></strong> A) [H<sub>3</sub>O<sup>+</sup>] < [OH<sup>-</sup>] B) [H<sub>3</sub>O<sup>+</sup>]<sub> </sub>= [HCO<sub>2</sub>H] C) [H<sub>3</sub>O<sup>+</sup>]<sub> </sub>= [HCO<sub>2</sub><sup>-</sup>] D) [H<sub>3</sub>O<sup>+</sup>] = [H<sub>2</sub>O] E) [OH<sup>-</sup>] = [H<sub>2</sub>O]](https://storage.examlex.com/TB9692/11ee9da3_9df0_ade9_9ff6_9f249b101645_TB9692_11.jpg) HCO2-(aq) + H3O+(aq) Ka = 1.8 x 10-4
HCO2-(aq) + H3O+(aq) Ka = 1.8 x 10-4A) [H3O+] < [OH-]
B) [H3O+] = [HCO2H]
C) [H3O+] = [HCO2-]
D) [H3O+] = [H2O]
E) [OH-] = [H2O]

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following isn't true for the H3O+ ion concentration in a
0)0100 M solution of acetic acid (Ka = 1.8 x 10-5)?
A) It is less than the H3O+ concentration in an 0.0100 M solution of HCl.
B) It is equal to the OAc- ion concentration.
C) It is equal to the OH- ion concentration.
D) The pH of the solution is less than 7.0.
E) None of the above are true.
0)0100 M solution of acetic acid (Ka = 1.8 x 10-5)?
A) It is less than the H3O+ concentration in an 0.0100 M solution of HCl.
B) It is equal to the OAc- ion concentration.
C) It is equal to the OH- ion concentration.
D) The pH of the solution is less than 7.0.
E) None of the above are true.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the approximate pH of a 0.10 M lactic acid solution.
(Ka = 8.4 x 10-4)
A) 2.0
B) 3.0
C) 4.0
D) 7.0
E) 12.0
(Ka = 8.4 x 10-4)
A) 2.0
B) 3.0
C) 4.0
D) 7.0
E) 12.0

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate the approximate pH of a 0.100 M solution of formic acid (HCO2H) in water. (HCO2H: Ka = 1.8 x 10-4)
A) 2.4
B) 3.7
C) 4.7
D) 7
E) 11.6
A) 2.4
B) 3.7
C) 4.7
D) 7
E) 11.6

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
57
Hydrogen peroxide has been used as a bleach to change hair color, as a disinfectant to treat wounds and as a rocket fuel. It is also a weak acid. Calculate the pH of a 0.018 M H2O2 solution. (H2O2: Ka = 2.2 x 10-12)
A) 0.6
B) 6.7
C) 7.3
D) 11.7
E) 13.4
A) 0.6
B) 6.7
C) 7.3
D) 11.7
E) 13.4

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
58
A 0.10 M solution of a weak acid, HA, is found to be 1.50% ionized. Calculate Ka for this acid.
A) 1.5
B) 2.3 x 10-1
C) 2.3 x 10-5
D) 2.3 x 10-6
E) none of the above
A) 1.5
B) 2.3 x 10-1
C) 2.3 x 10-5
D) 2.3 x 10-6
E) none of the above

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
59
A solution is prepared by dissolving 1.0 x 10-4 moles of HOBr in water and diluting to 1.00 L. What is the pH of this solution? (HOBr: Ka = 2 x 10-9)
A) 1.3
B) 5.3
C) 6.3
D) 8.7
E) 12.7
A) 1.3
B) 5.3
C) 6.3
D) 8.7
E) 12.7

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following groups contains salts that all form basic solutions in water?
A) NaNO3, NH4CN, NaOAc, NH4Cl
B) Na2CO3, KCl, NaOAc, NH4Cl
C) Na2CO3, NaF, NaOAc, NaCN
D) NaHCO3, NaF, NH4Cl, Na2SO3
E) none of the above
A) NaNO3, NH4CN, NaOAc, NH4Cl
B) Na2CO3, KCl, NaOAc, NH4Cl
C) Na2CO3, NaF, NaOAc, NaCN
D) NaHCO3, NaF, NH4Cl, Na2SO3
E) none of the above

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following compounds aren't acids in water?
(I) NH4Cl (II) HNO3 (III) NH3 (IV) HI (V) K2S
A) I and II
B) II and III
C) III and V
D) I, II and IV
E) all of the above are acids in water
(I) NH4Cl (II) HNO3 (III) NH3 (IV) HI (V) K2S
A) I and II
B) II and III
C) III and V
D) I, II and IV
E) all of the above are acids in water

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
62
Kb for methylamine (CH3NH2) is the equilibrium constant for which reaction?
A) CH3NH2 + H2O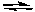 CH3NH- + H3O+
CH3NH- + H3O+
B) CH3NH2 + H2O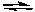 CH3NH3+ + OH-
CH3NH3+ + OH-
C) CH3NH2 + H3O+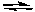 CH3NH3+ + H2O
CH3NH3+ + H2O
D) CH3NH2 + OH-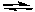 CH3NH- + H2O
CH3NH- + H2O
E) None of the above
A) CH3NH2 + H2O
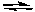 CH3NH- + H3O+
CH3NH- + H3O+B) CH3NH2 + H2O
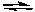 CH3NH3+ + OH-
CH3NH3+ + OH-C) CH3NH2 + H3O+
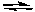 CH3NH3+ + H2O
CH3NH3+ + H2OD) CH3NH2 + OH-
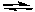 CH3NH- + H2O
CH3NH- + H2OE) None of the above

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
63
What is the Kb for methylamine (CH3NH2) if a 0.10 M solution of this weak base is 6.8% ionized?
A) 5.0 x 10-4
B) 0.068
C) 4.6 x 10-5
D) 2.0 x 103
E) 1.0
A) 5.0 x 10-4
B) 0.068
C) 4.6 x 10-5
D) 2.0 x 103
E) 1.0

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following won't produce an acidic solution when dissolved in water?
A) HF
B) NH4Cl
C) CH3CO2H
D) CH3CO2Na
E) HClO4
A) HF
B) NH4Cl
C) CH3CO2H
D) CH3CO2Na
E) HClO4

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
65
Sodium fluoride, NaF (a soluble salt), is dissolved in water. Which one of the following correctly describes the relationships between the concentrations of the species present at equilibrium?
A) [ F- ] < [HF] and [Na+]![<strong>Sodium fluoride, NaF (a soluble salt), is dissolved in water. Which one of the following correctly describes the relationships between the concentrations of the species present at equilibrium? </strong> A) [ F<sup>-</sup> ] < [HF] and [Na<sup>+</sup>] [ F<sup>-</sup> ] B) [F <sup>-</sup> ] [Na<sup>+</sup>] and [H<sub>3</sub>O<sup>+</sup>] [F <sup>-</sup> ] C) [ F <sup>-</sup> ] 0 [HF] and [Na<sup>+</sup>] < [ F <sup>-</sup> ] D) [OH <sup>-</sup> ] < [HF] and [Na<sup>+</sup>] [ F <sup>-</sup> ] E) none of these](https://storage.examlex.com/TB9692/11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11.jpg) [ F- ]
[ F- ]
B) [F - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Na+] and [H3O+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [F - ]
C) [ F - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 0 [HF] and [Na+] < [ F - ]
D) [OH - ] < [HF] and [Na+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [ F - ]
E) none of these
A) [ F- ] < [HF] and [Na+]
![<strong>Sodium fluoride, NaF (a soluble salt), is dissolved in water. Which one of the following correctly describes the relationships between the concentrations of the species present at equilibrium? </strong> A) [ F<sup>-</sup> ] < [HF] and [Na<sup>+</sup>] [ F<sup>-</sup> ] B) [F <sup>-</sup> ] [Na<sup>+</sup>] and [H<sub>3</sub>O<sup>+</sup>] [F <sup>-</sup> ] C) [ F <sup>-</sup> ] 0 [HF] and [Na<sup>+</sup>] < [ F <sup>-</sup> ] D) [OH <sup>-</sup> ] < [HF] and [Na<sup>+</sup>] [ F <sup>-</sup> ] E) none of these](https://storage.examlex.com/TB9692/11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11.jpg) [ F- ]
[ F- ]B) [F - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Na+] and [H3O+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [F - ]
C) [ F - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 0 [HF] and [Na+] < [ F - ]
D) [OH - ] < [HF] and [Na+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [ F - ]
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
66
The salt NaF is dissolved in water. Which of the following relationships would be true for the species in solution?
A) [Na+]![<strong>The salt NaF is dissolved in water. Which of the following relationships would be true for the species in solution?</strong> A) [Na<sup>+</sup>] [F <sup>-</sup> ] >> [HF] [H<sub>3</sub>O<sup>+</sup>] >> [OH <sup>-</sup> ] B) [Na<sup>+</sup>] [F <sup>-</sup> ] >> [HF] [OH <sup>-</sup> ] >> [H<sub>3</sub>O<sup>+</sup>] C) [Na<sup>+</sup>] >> [F <sup>-</sup> ] [HF] [OH <sup>-</sup> ] >> [H<sub>3</sub>O<sup>+</sup>] D) [Na<sup>+</sup>] [HF] >> [F <sup>-</sup> ] [OH <sup>-</sup> ] >> [H<sub>3</sub>O<sup>+</sup>] E) none of these](https://storage.examlex.com/TB9692/11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11.jpg) [F - ] >> [HF] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+] >> [OH - ]
[F - ] >> [HF] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+] >> [OH - ]
B) [Na+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [F - ] >> [HF] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] >> [H3O+]
C) [Na+] >> [F - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [HF] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] >> [H3O+]
D) [Na+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [HF] >> [F - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] >> [H3O+]
E) none of these
A) [Na+]
![<strong>The salt NaF is dissolved in water. Which of the following relationships would be true for the species in solution?</strong> A) [Na<sup>+</sup>] [F <sup>-</sup> ] >> [HF] [H<sub>3</sub>O<sup>+</sup>] >> [OH <sup>-</sup> ] B) [Na<sup>+</sup>] [F <sup>-</sup> ] >> [HF] [OH <sup>-</sup> ] >> [H<sub>3</sub>O<sup>+</sup>] C) [Na<sup>+</sup>] >> [F <sup>-</sup> ] [HF] [OH <sup>-</sup> ] >> [H<sub>3</sub>O<sup>+</sup>] D) [Na<sup>+</sup>] [HF] >> [F <sup>-</sup> ] [OH <sup>-</sup> ] >> [H<sub>3</sub>O<sup>+</sup>] E) none of these](https://storage.examlex.com/TB9692/11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11.jpg) [F - ] >> [HF] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+] >> [OH - ]
[F - ] >> [HF] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+] >> [OH - ]B) [Na+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [F - ] >> [HF] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] >> [H3O+]
C) [Na+] >> [F - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [HF] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] >> [H3O+]
D) [Na+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [HF] >> [F - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] >> [H3O+]
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
67
When NH4Cl is dissolved in water the following species will be present: NH4+, Cl - OH - , H3O+, and NH3. Which of the following represents the correct relationship between the species in solution?
A) [NH4+]![<strong>When NH<sub>4</sub>Cl is dissolved in water the following species will be present: NH<sub>4</sub><sup>+</sup>, Cl <sup>-</sup> OH <sup>-</sup> , H<sub>3</sub>O<sup>+</sup>, and NH<sub>3</sub>. Which of the following represents the correct relationship between the species in solution?</strong> A) [NH<sub>4</sub><sup>+</sup>] [Cl <sup>-</sup> ] > [OH <sup>-</sup> ] > [NH<sub>3</sub>] > [H<sub>3</sub>O<sup>+</sup>] B) [NH<sub>4</sub><sup>+</sup>] [Cl <sup>-</sup> ] > [NH<sub>3</sub>] [H<sub>3</sub>O<sup>+</sup>] > [OH <sup>-</sup> ] C) [NH<sub>4</sub><sup>+</sup>] [Cl <sup>-</sup> ] > [NH<sub>3</sub>] [OH <sup>-</sup> ] > [H<sub>3</sub>O<sup>+</sup>] D) [NH<sub>3</sub>] [OH <sup>-</sup> ] [Cl <sup>-</sup> ] > [NH<sub>4</sub><sup>+</sup>] [H<sub>3</sub>O<sup>+</sup>] E) [NH<sub>3</sub>] [H<sub>3</sub>O<sup>+</sup>] [Cl <sup>-</sup> ] > [NH<sub>4</sub><sup>+</sup>] [OHF <sup>-</sup> ]](https://storage.examlex.com/TB9692/11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11.jpg) [Cl - ] > [OH - ] > [NH3] > [H3O+]
[Cl - ] > [OH - ] > [NH3] > [H3O+]
B) [NH4+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Cl - ] > [NH3] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+] > [OH - ]
C) [NH4+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Cl - ] > [NH3] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] > [H3O+]
D) [NH3] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Cl - ] > [NH4+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+]
E) [NH3] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Cl - ] > [NH4+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OHF - ]
A) [NH4+]
![<strong>When NH<sub>4</sub>Cl is dissolved in water the following species will be present: NH<sub>4</sub><sup>+</sup>, Cl <sup>-</sup> OH <sup>-</sup> , H<sub>3</sub>O<sup>+</sup>, and NH<sub>3</sub>. Which of the following represents the correct relationship between the species in solution?</strong> A) [NH<sub>4</sub><sup>+</sup>] [Cl <sup>-</sup> ] > [OH <sup>-</sup> ] > [NH<sub>3</sub>] > [H<sub>3</sub>O<sup>+</sup>] B) [NH<sub>4</sub><sup>+</sup>] [Cl <sup>-</sup> ] > [NH<sub>3</sub>] [H<sub>3</sub>O<sup>+</sup>] > [OH <sup>-</sup> ] C) [NH<sub>4</sub><sup>+</sup>] [Cl <sup>-</sup> ] > [NH<sub>3</sub>] [OH <sup>-</sup> ] > [H<sub>3</sub>O<sup>+</sup>] D) [NH<sub>3</sub>] [OH <sup>-</sup> ] [Cl <sup>-</sup> ] > [NH<sub>4</sub><sup>+</sup>] [H<sub>3</sub>O<sup>+</sup>] E) [NH<sub>3</sub>] [H<sub>3</sub>O<sup>+</sup>] [Cl <sup>-</sup> ] > [NH<sub>4</sub><sup>+</sup>] [OHF <sup>-</sup> ]](https://storage.examlex.com/TB9692/11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11.jpg) [Cl - ] > [OH - ] > [NH3] > [H3O+]
[Cl - ] > [OH - ] > [NH3] > [H3O+]B) [NH4+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Cl - ] > [NH3] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+] > [OH - ]
C) [NH4+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Cl - ] > [NH3] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] > [H3O+]
D) [NH3] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OH - ] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Cl - ] > [NH4+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+]
E) [NH3] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [H3O+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [Cl - ] > [NH4+] 11ee9f02_5f5c_eb1b_9fac_d5ba514ecb51_TB9692_11 [OHF - ]

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
68
What is the value of Kb for the formate (HCO2-) ion if Ka for formic acid (HCO2H) is 1.8x10-4?
A) Kb = Kw x Ka
B) Kb = Ka/Kw
C) Kb = Kw/Ka
D) Kb = Kw + Ka
E) Kb = Kw - Ka
A) Kb = Kw x Ka
B) Kb = Ka/Kw
C) Kb = Kw/Ka
D) Kb = Kw + Ka
E) Kb = Kw - Ka

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
69
The base-ionization constants for three bases, A(OH), B(OH), and C(OH), are 1.8 x 10-5, 4.4 x 10-4, and 7.4 x 10-4, respectively. Solutions of the bases having equal molarity have pH values that decrease in the following order:
A) A > B > C
B) B > C > A
C) C > B > A
D) A > C > B
E) B > A > C
A) A > B > C
B) B > C > A
C) C > B > A
D) A > C > B
E) B > A > C

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
70
There are many ways of "fluoridating" water. One approach involves adding a salt of the fluoride ion, such as NaF. Calculate the pH of a 0.15 M NaF solution. (HF: Ka = 7.2 x 10-4)
A) 2
B) 5.8
C) 8.2
D) 10.9
E) 12
A) 2
B) 5.8
C) 8.2
D) 10.9
E) 12

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
71
Ammonia and the ammonium ion form a conjugate acid-base pair.
NH3(aq) + H2O(l)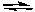 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
Calculate the pH of 0.10 M NH3 if Ka for the NH4+ ion is 5.6 x 10-10.
A) 4.7
B) 5.1
C) 5.7
D) 8.9
E) 11.1
NH3(aq) + H2O(l)
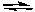 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)Calculate the pH of 0.10 M NH3 if Ka for the NH4+ ion is 5.6 x 10-10.
A) 4.7
B) 5.1
C) 5.7
D) 8.9
E) 11.1

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
72
Calculate the pH of a solution prepared by dissolving 1.00 x 10-2 moles of sodium hypochlorite (NaOCl) in enough water to produce a 1.00 L of solution. Hypochlorous acid is a weak monoprotic acid with Ka = 3.2 x 10-8.
A) 4.3
B) 4.7
C) 7.5
D) 9.3
E) 9.7
A) 4.3
B) 4.7
C) 7.5
D) 9.3
E) 9.7

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
73
What is the OH- ion concentration in a 0.200 M NaOAc solution?
(HOAc: Ka = 1.8 x 10-5)
A) 1.1 x 10-10
B) 5.7 x 10-10
C) 1.8 x 10-6
D) 1.1 x 10-5
E) 1.3 x 10-3
(HOAc: Ka = 1.8 x 10-5)
A) 1.1 x 10-10
B) 5.7 x 10-10
C) 1.8 x 10-6
D) 1.1 x 10-5
E) 1.3 x 10-3

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
74
What is the H3O+ ion concentration in a 0.10 M NH3 solution?
(Kb = 1.8 x 10-5)
A) 7.5 x 10-10
B) 3.0 x 10-10
C) 1.8 x 10-6
D) 1.3 x 10-3
E) none of these
(Kb = 1.8 x 10-5)
A) 7.5 x 10-10
B) 3.0 x 10-10
C) 1.8 x 10-6
D) 1.3 x 10-3
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
75
HA is a weak monoprotic acid with Ka = 2.4 x 10-6. What is [OH-] in a 0.300 M solution of the salt, NaA?
A) 5.8 x 10-12
B) 2.4 x 10-10
C) 3.5 x 10-5
D) 6.5 x 10-5
E) 1.6 x 10-3
A) 5.8 x 10-12
B) 2.4 x 10-10
C) 3.5 x 10-5
D) 6.5 x 10-5
E) 1.6 x 10-3

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following solutions would have a pH greater than 7.0?
A) NH4Cl
B) NaCl
C) CH3COOH
D) CH3COONa
E) HCl
A) NH4Cl
B) NaCl
C) CH3COOH
D) CH3COONa
E) HCl

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following solutions has a pH greater than 7.0?
A) 0.10 M NH4Cl
B) 0.10 M NH4NO3
C) 0.10 M HCN
D) 0.10 M NaCN
E) none of these
A) 0.10 M NH4Cl
B) 0.10 M NH4NO3
C) 0.10 M HCN
D) 0.10 M NaCN
E) none of these

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
78
Predict the products of the following acid-base reaction.
NH4Cl(aq) + NaSH(aq)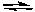
NH4Cl(aq) + NaSH(aq)

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following solutions has the highest pH?
A) 0.3 M Na2CO3
B) 1 M HOAc
C) 0.3 M NH4Cl
D) water
E) 10-3 M HCl
A) 0.3 M Na2CO3
B) 1 M HOAc
C) 0.3 M NH4Cl
D) water
E) 10-3 M HCl

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
80
The addition of sodium formate (HCO2Na) to a solution containing formic acid (HCO2H: Ka = 1.8 x 10-4) will cause
A) the pH to increase.
B) the pH to decrease.
C) no change in pH.
D) a change in the pH, the direction of which cannot be predicted.
A) the pH to increase.
B) the pH to decrease.
C) no change in pH.
D) a change in the pH, the direction of which cannot be predicted.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck


