Exam 11: Acids and Bases
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
What is the [H3O+] concentration of a solution with a pH of 8.75?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
A
The dihydrogen phosphate ion, H2PO4 - , can behave as both an acid and as a base. What is the chemical formula of the conjugate acid of dihydrogen phosphate? What is the chemical formula of the conjugate base of dihydrogen phosphate?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
A
A strong acid is added to water. Which of the following will be true?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
E
What is the OH- ion concentration in a 0.200 M NaOAc solution?
(HOAc: Ka = 1.8 x 10-5)
(Multiple Choice)
4.9/5  (33)
(33)
What is the Kb for methylamine (CH3NH2) if a 0.10 M solution of this weak base is 6.8% ionized?
(Multiple Choice)
4.7/5  (39)
(39)
What is the pH of a 0.10 M succinic acid, H2Sc, solution if we assume that H2Sc is a weak acid that undergoes stepwise dissociation?
H2Sc(aq) + H2O(l)  H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
HSc-(aq) + H2O(l)
H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
HSc-(aq) + H2O(l)  H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following is the conjugate base of a strong acid?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following statements should be true for a 1.0x 10 - 4 M solution of a strong acid HBr?
(Multiple Choice)
4.9/5  (42)
(42)
A solution is prepared by dissolving 1.0 x 10-4 moles of HOBr in water and diluting to 1.00 L. What is the pH of this solution? (HOBr: Ka = 2 x 10-9)
(Multiple Choice)
4.9/5  (36)
(36)
Using data from the previous problem, what fraction of the HSc- formed in the first step of the dissociation of succinic acid goes on to dissociate to form Sc2- ions in the second step, and what does this lead us to conclude?
(Multiple Choice)
5.0/5  (35)
(35)
If the strength of the following acids increases from left to right
CH3NH2 < CH3NH3+ < C6H5NH3 + < C6H5CO2H < HCl
The order of increasing base strength, reading from left to right, must be:
(Multiple Choice)
4.9/5  (40)
(40)
When NH4Cl is added to a 0.10 M NH3 solution, the pH of this solution
(Multiple Choice)
4.8/5  (38)
(38)
What is the [HOAc]/[OAc-] ratio in an acetic acid/sodium acetate buffer at pH = 4.9? (HOAc: Ka = 1.8 x 10-5)
(Multiple Choice)
4.7/5  (34)
(34)
What can be correctly concluded from the fact that the following acid-base reaction proceeds to the right, as written?
NH2-(aq) + HSO4-(aq) 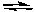 NH3(aq) + SO42-(aq)
NH3(aq) + SO42-(aq)
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following would be the strongest Brønsted base?
(Multiple Choice)
4.9/5  (43)
(43)
What is the H3O+ ion concentration in a solution that has a pH of 5.75?
(Multiple Choice)
4.8/5  (35)
(35)
A 0.10 M solution of a weak acid, HA, is found to be 1.50% ionized. Calculate Ka for this acid.
(Multiple Choice)
4.9/5  (37)
(37)
KHS can act as a weak acid or a weak base.
HS-(aq) + H2O(l)  S2-(aq) + H3O+(aq) Ka2 = 1.3 x 10-13
HS-(aq) + H2O(l)
S2-(aq) + H3O+(aq) Ka2 = 1.3 x 10-13
HS-(aq) + H2O(l)  H2S(aq) + OH-(aq) Kb2 = 1.0 x 10-7
Which of the following statements is correct?
H2S(aq) + OH-(aq) Kb2 = 1.0 x 10-7
Which of the following statements is correct?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)