Deck 10: An Introduction to Kinetics and Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 10: An Introduction to Kinetics and Equilibrium
1
The following reaction was carried out:
Ni(CO)4 + PPh3(g) PPh3Ni(CO)3 + CO
Over 200 seconds, the concentration of Ni(CO)4 dropped from 10 M to 2.5 M. What is the average rate of the reaction in M/s over this time period?
A) -0.038 M/s
B) 0.050 M/s
C) -0.050 M/s
D) 1.5 x 103 M/s
E) 0.038 M/s
Ni(CO)4 + PPh3(g) PPh3Ni(CO)3 + CO
Over 200 seconds, the concentration of Ni(CO)4 dropped from 10 M to 2.5 M. What is the average rate of the reaction in M/s over this time period?
A) -0.038 M/s
B) 0.050 M/s
C) -0.050 M/s
D) 1.5 x 103 M/s
E) 0.038 M/s
0.038 M/s
2
Describe the relationship between the rate constants kf and kr for the following one step reaction at equilibrium.
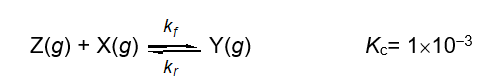
A) kf = kr
B) kf < kr
C) kf > kr
D) kf = kr = 0
E) none of these
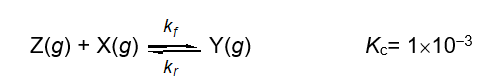
A) kf = kr
B) kf < kr
C) kf > kr
D) kf = kr = 0
E) none of these
kf < kr
3
For the reaction:
CO(g) + Cl2(g) COCl2(g)
COCl2(g)
Kf is 1x 107 M-1-s-1 and kr = 2 x 102 s-1.
What is the equilibrium constant for the reaction?
A) 2 x 10-5
B) 5
C) 5 x 104
D) 2 x 109
E) none of these
CO(g) + Cl2(g)
 COCl2(g)
COCl2(g)Kf is 1x 107 M-1-s-1 and kr = 2 x 102 s-1.
What is the equilibrium constant for the reaction?
A) 2 x 10-5
B) 5
C) 5 x 104
D) 2 x 109
E) none of these
5 x 104
4
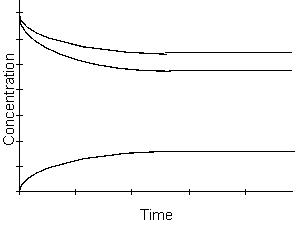
-Which of the following reactions could be described by the above diagram?
A) 2 N2O2(g)
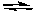 4 NO2(g) + O2(g)
4 NO2(g) + O2(g)B) H2(g) + CO2(g)
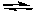 CO(g) + H2O(g)
CO(g) + H2O(g)C) PCl5(g)
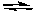 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)D) 2 NO(g) + Br2(g)
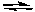 2 NOBr(g)
2 NOBr(g)E) 2 O3(g)
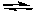 3 O2(g)
3 O2(g)
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5

-What happens with respect to time to the products of this reaction in the kinetic region of the graph above?
A) the products increase
B) the products decrease
C) the products remain constant
D) cannot be determined from the graph

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6

-What happens to the rate of the reaction as time passes in the equilibrium region of the graph above?
A) the rate increases
B) the rate decreases
C) the rate remains constant
D) cannot be determined from the graph

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7

-From the graph above, which of the following is true when equilibrium has been reached?
A) The concentrations of the reactants are larger than the concentration of the products.
B) The concentrations of the products are larger than the concentration of the reactants.
C) The concentrations of the reactants and products are equal.
D) This cannot be determined from the graph.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8

-Which of the following will be true concerning the Kc of the reaction described by the graph above?
A) Kc < 1
B) Kc > 1
C) Kc = 1
D) Kc cannot be determined from the graph

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
For the following reaction:
2 NOCl(g)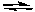 2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO2) = 0.989 M at some moment in time, which of the following would be true?
A) The rate of the forward reaction would be exactly equal to the rate of the reverse reaction.
B) Neither the forward nor the reverse reaction would occur because the reaction would be at equilibrium.
C) The rate of the forward reaction would be faster than the reverse reaction.
D) The rate of the reverse reaction would be faster than the forward reaction.
E) There is no relationship between these concentrations and the rate of either the forward or the reverse reaction.
2 NOCl(g)
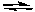 2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO2) = 0.989 M at some moment in time, which of the following would be true?
A) The rate of the forward reaction would be exactly equal to the rate of the reverse reaction.
B) Neither the forward nor the reverse reaction would occur because the reaction would be at equilibrium.
C) The rate of the forward reaction would be faster than the reverse reaction.
D) The rate of the reverse reaction would be faster than the forward reaction.
E) There is no relationship between these concentrations and the rate of either the forward or the reverse reaction.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following graphs best represents the relationship between the concentration of reactants and products with respect to time for the following chemical reaction?
2 NO2(g) 2 NO(g) + O2(g)
2 NO(g) + O2(g)
A)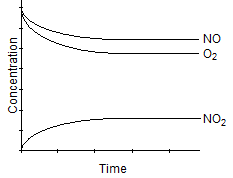
B)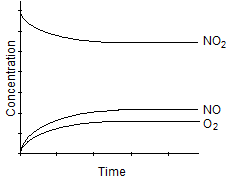
C)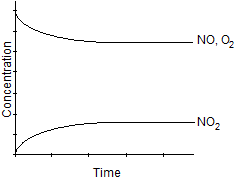
D)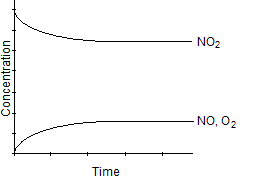
E)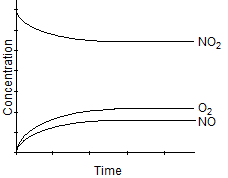
2 NO2(g)
 2 NO(g) + O2(g)
2 NO(g) + O2(g)A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
Use the following graph for answering
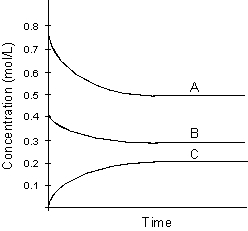
-Using the above graph determine the balanced chemical equation.
A) 3 A + B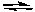 2 C
2 C
B) 5 A + 3 B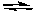 2 C
2 C
C) 2 C + 3 B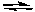 5 A
5 A
D) A + B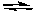 C
C
E) none of these

-Using the above graph determine the balanced chemical equation.
A) 3 A + B
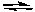 2 C
2 CB) 5 A + 3 B
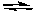 2 C
2 CC) 2 C + 3 B
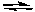 5 A
5 AD) A + B
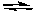 C
CE) none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
Use the following graph for answering

-Which of the following will be true of the equilibrium constant, Kc, for the reaction described in the above graph?
A) Kc 1
B) Kc 0
C) Kc < 1
D) Kc > 1
E) cannot be determined from graph

-Which of the following will be true of the equilibrium constant, Kc, for the reaction described in the above graph?
A) Kc 1
B) Kc 0
C) Kc < 1
D) Kc > 1
E) cannot be determined from graph

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
At equilibrium, ______________.
A) all chemical processes have ceased.
B) the rate of the forward reaction equals that of the reverse.
C) the rate constant for the forward reaction equals that of the reverse.
D) the rate of the forward reaction equals that of the reverse the rate constant for the forward reaction equals that of the reverse.
E) none of the above
A) all chemical processes have ceased.
B) the rate of the forward reaction equals that of the reverse.
C) the rate constant for the forward reaction equals that of the reverse.
D) the rate of the forward reaction equals that of the reverse the rate constant for the forward reaction equals that of the reverse.
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following graphs best represents the relationship between the concentration of reactants and products with respect to time for the following chemical reaction?
2 H2(g) + O2(g)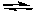 2 H2O(g)
2 H2O(g)
A)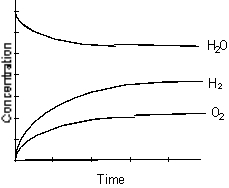
B)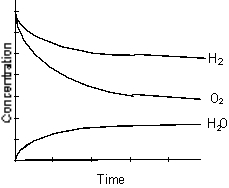
C)
D)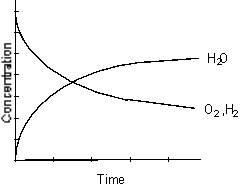
E)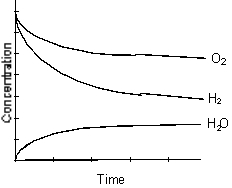
2 H2(g) + O2(g)
 2 H2O(g)
2 H2O(g)A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
Assume that the equilibrium constants for the following reactions are known.
2 SO2(g) + O2(g) 2 SO3(g) K1
2 SO3(g) K1
2 CO(g) + O2(g)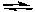 2 CO2(g) K2
2 CO2(g) K2
What is the equilibrium constant for the following reaction?
2 SO2(g) + 2 CO2(g)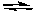 2 SO3(g) + 2 CO(g)
2 SO3(g) + 2 CO(g)
A) K = K1 x K2
B) K = K1/K2
C) K = K2/K1
D) K = K1 + K2
E) K = K1 - K2
2 SO2(g) + O2(g)
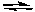 2 SO3(g) K1
2 SO3(g) K12 CO(g) + O2(g)
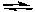 2 CO2(g) K2
2 CO2(g) K2What is the equilibrium constant for the following reaction?
2 SO2(g) + 2 CO2(g)
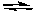 2 SO3(g) + 2 CO(g)
2 SO3(g) + 2 CO(g)A) K = K1 x K2
B) K = K1/K2
C) K = K2/K1
D) K = K1 + K2
E) K = K1 - K2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
Which is the correct equilibrium constant expression for the reaction:
2 SO2(g) + O2(g)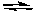 2 SO3(g)
2 SO3(g)
A)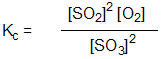
B)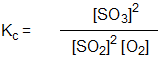
C)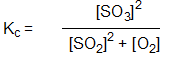
D)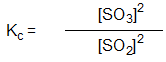
2 SO2(g) + O2(g)
 2 SO3(g)
2 SO3(g)A)
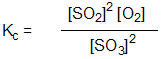
B)
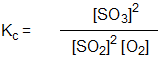
C)
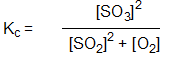
D)
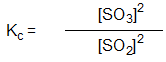

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
Assume that the equilibrium constant for the following reaction is known.
2 NO2(g) 2 NO(g) + O2(g) K1 = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) K1 = 7.4 x 10-16 (at 25°C)
What is the correct value of the equilibrium constant for the opposite reaction?
2 NO(g) + O2(g)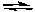 2 NO2(g) K2 = ?
2 NO2(g) K2 = ?
A) K2 = K1 = 7.4 x 10-16
B) K2 = 1/K1 = 1.4 x 1015
C) K2 = K1(RT) = 1.8 x 10-14
D) K2 = K1(RT)-1 = 3.0 x 10-17
E) impossible to determine from this information
2 NO2(g)
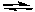 2 NO(g) + O2(g) K1 = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) K1 = 7.4 x 10-16 (at 25°C)What is the correct value of the equilibrium constant for the opposite reaction?
2 NO(g) + O2(g)
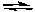 2 NO2(g) K2 = ?
2 NO2(g) K2 = ?A) K2 = K1 = 7.4 x 10-16
B) K2 = 1/K1 = 1.4 x 1015
C) K2 = K1(RT) = 1.8 x 10-14
D) K2 = K1(RT)-1 = 3.0 x 10-17
E) impossible to determine from this information

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
For the reaction shown below, at equilibrium
I2(g) + Br2(g)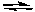 2IBr(g) Kc = 1.1 x 102
2IBr(g) Kc = 1.1 x 102
A) Reactants and products will be present in approximately equal concentrations.
B) Reactants will be favored.
C) Products will be favored.
D) Insufficient information is given to decide.
E) It is impossible for the reaction to reach equilibrium.
I2(g) + Br2(g)
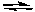 2IBr(g) Kc = 1.1 x 102
2IBr(g) Kc = 1.1 x 102A) Reactants and products will be present in approximately equal concentrations.
B) Reactants will be favored.
C) Products will be favored.
D) Insufficient information is given to decide.
E) It is impossible for the reaction to reach equilibrium.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
Based on the information given in the previous problem, the equilibrium constant for the following reaction would be:
2IBr(g)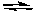 I2(g) + Br2(g)
I2(g) + Br2(g)
A) -1.1 x 102
B) 9.1 x 10-3
C) 10.5
D) 1.1 x 10-2
E) none of the above
2IBr(g)
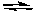 I2(g) + Br2(g)
I2(g) + Br2(g)A) -1.1 x 102
B) 9.1 x 10-3
C) 10.5
D) 1.1 x 10-2
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
Under which set of conditions must the following reaction always proceed to the right in order to reach equilibrium?
H2(g) + I2(g)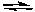 2 HI(g)
2 HI(g)
A) Qc < 1
B) Qc > 1
C) Qc = 1
D) Qc > Kc
E) none of these
H2(g) + I2(g)
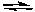 2 HI(g)
2 HI(g)A) Qc < 1
B) Qc > 1
C) Qc = 1
D) Qc > Kc
E) none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following initial concentrations of reactants and products will cause the reaction to proceed to the right in order to establish equilibrium?
2 NO2(g)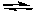 2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200
2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200  C)
C)
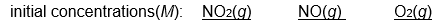
A)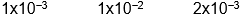
B)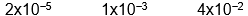
C)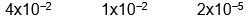
D)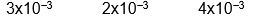
E) none of these
2 NO2(g)
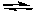 2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200
2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200  C)
C)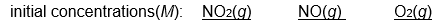
A)
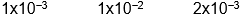
B)
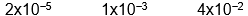
C)
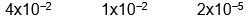
D)
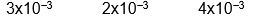
E) none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
If at any moment in time, we find that Qc is larger than Kc for the reaction
Cl2(g) + 3 F2(g) 2 ClF3(g)
2 ClF3(g)
We can conclude that:
A) The reaction is at equilibrium.
B) The reaction cannot reach equilibrium.
C) The reaction must shift to the right to reach equilibrium.
D) The reaction must shift to the left to reach equilibrium.
E) None of the above are possible.
Cl2(g) + 3 F2(g)
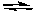 2 ClF3(g)
2 ClF3(g)We can conclude that:
A) The reaction is at equilibrium.
B) The reaction cannot reach equilibrium.
C) The reaction must shift to the right to reach equilibrium.
D) The reaction must shift to the left to reach equilibrium.
E) None of the above are possible.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
Assume that the reaction quotient, Qc, for the following reaction at 25°C is 1.0 x 10-8
2 NO2(g)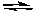 2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
From this we can conclude:
A) The reaction is at equilibrium.
B) Equilibrium could be reached by adding enough NO or O2 to the system.
C) The reaction must proceed from left to right to reach equilibrium.
D) The reaction must proceed from right to left to reach equilibrium.
E) The reaction can never reach equilibrium.
2 NO2(g)
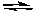 2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)From this we can conclude:
A) The reaction is at equilibrium.
B) Equilibrium could be reached by adding enough NO or O2 to the system.
C) The reaction must proceed from left to right to reach equilibrium.
D) The reaction must proceed from right to left to reach equilibrium.
E) The reaction can never reach equilibrium.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
If the equilibrium constant for the following reaction is Kc = 1 x 102
2 C2H6(g) + 7 O2(g)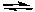 4 CO2(g) + 6 H2O(g)
4 CO2(g) + 6 H2O(g)
And all the concentrations were initially 0.10 M, we can predict that the reaction
A) is at equilibrium initially.
B) must shift from left to right to reach equilibrium.
C) must shift from right to left to reach equilibrium.
D) cannot reach equilibrium.
E) cannot be determined unless we have the information necessary to calculate Qc for the reaction.
2 C2H6(g) + 7 O2(g)
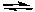 4 CO2(g) + 6 H2O(g)
4 CO2(g) + 6 H2O(g)And all the concentrations were initially 0.10 M, we can predict that the reaction
A) is at equilibrium initially.
B) must shift from left to right to reach equilibrium.
C) must shift from right to left to reach equilibrium.
D) cannot reach equilibrium.
E) cannot be determined unless we have the information necessary to calculate Qc for the reaction.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
For the following reaction:
2 NOCl(g)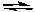 2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO) = 0.989
M at some moment in time, we can conclude that
A) the reaction is at equilibrium.
B) some of the NOCl must be converted to NO and Cl2 to reach equilibrium.
C) some of the NO and Cl2 must be converted to NOCl to reach equilibrium.
D) it is impossible to determine which way the reaction must go to reach equilibrium.
E) the reaction can never reach equilibrium.
2 NOCl(g)
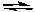 2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO) = 0.989
M at some moment in time, we can conclude that
A) the reaction is at equilibrium.
B) some of the NOCl must be converted to NO and Cl2 to reach equilibrium.
C) some of the NO and Cl2 must be converted to NOCl to reach equilibrium.
D) it is impossible to determine which way the reaction must go to reach equilibrium.
E) the reaction can never reach equilibrium.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
Suppose that the reaction quotient, Qc, for a chemical reaction was found to be close to unity (Qc 1). Given this information we can accurately predict
A) that the reaction will proceed in a direction to generate more product.
B) that the reaction will proceed in a direction to use up product.
C) that the reaction is close to equilibrium.
D) nothing about the direction in which the reaction will proceed without additional information.
E) none of the above.
A) that the reaction will proceed in a direction to generate more product.
B) that the reaction will proceed in a direction to use up product.
C) that the reaction is close to equilibrium.
D) nothing about the direction in which the reaction will proceed without additional information.
E) none of the above.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
For the following reaction 2 NO2(g) 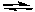 2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
If (NO2) = 0.10 M, (NO) = 0.010 M, and (O2) = 1.0 x 10-5 M, we can correctly predict
A) that the reaction is at equilibrium.
B) that the reaction must shift to the left to reach equilibrium.
C) that the reaction must shift to the right to reach equilibrium.
D) nothing related to the question as to whether the reaction is at equilibrium.
E) nothing related to the question as to which direction the reaction has to shift to reach equilibrium.
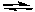 2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)If (NO2) = 0.10 M, (NO) = 0.010 M, and (O2) = 1.0 x 10-5 M, we can correctly predict
A) that the reaction is at equilibrium.
B) that the reaction must shift to the left to reach equilibrium.
C) that the reaction must shift to the right to reach equilibrium.
D) nothing related to the question as to whether the reaction is at equilibrium.
E) nothing related to the question as to which direction the reaction has to shift to reach equilibrium.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
The following reaction was carried out at 250C with the initial concentration of NO2 being 0.70 M and no NO(g) or O2(g) initially present. At equilibrium the NO2 concentration was found to be 0.28 M. Calculate Kc for the reaction.
2NO2(g)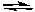 2NO(g) + O2(g)
2NO(g) + O2(g)
A) 0.47
B) 0.94
C) 1.9
D) 0.14
E) 2.1
2NO2(g)
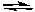 2NO(g) + O2(g)
2NO(g) + O2(g)A) 0.47
B) 0.94
C) 1.9
D) 0.14
E) 2.1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
For the reaction below, 0.0500 moles of ethyl acetate are allowed to react with 0.200 moles of water. At equilibrium it is found that 0.0300 moles of acetic acid have been produced. How many moles of ethyl acetate remain at equilibrium?
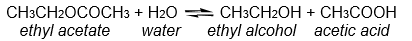
A) 0.0100 moles
B) 0.0300 moles
C) 0.0500 moles
D) 0.170 moles
E) none of these
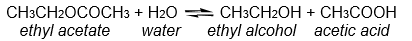
A) 0.0100 moles
B) 0.0300 moles
C) 0.0500 moles
D) 0.170 moles
E) none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
A 0.100 M sample of SO2 reacts with 0.060 M O2 to produce SO3 by the reaction shown below. There was no SO3 present initially. The concentration of O2 measured at equilibrium is found to be 0.020 M. What are the equilibrium concentrations of SO2 and SO3?
2 SO2(g) + O2(g)![<strong>A 0.100 M sample of SO<sub>2</sub> reacts with 0.060 M O<sub>2</sub> to produce SO<sub>3</sub> by the reaction shown below. There was no SO<sub>3</sub> present initially. The concentration of O<sub>2</sub> measured at equilibrium is found to be 0.020 M. What are the equilibrium concentrations of SO<sub>2</sub> and SO<sub>3</sub>? 2 SO<sub>2</sub>(g) + O<sub>2</sub>(g) 2 SO<sub>3</sub>(g)</strong> A) [SO<sub>2</sub>] = 0.040M, [SO<sub>3</sub>] = 0.060M B) [SO<sub>2</sub>] = 0.080M, [SO<sub>3</sub>] = 0.080M C) [SO<sub>2</sub>] = 0.080M, [SO<sub>3</sub>] = 0.120M D) [SO<sub>2</sub>] = 0.020M, [SO<sub>3</sub>] = 0.080M E) none of these](https://storage.examlex.com/TB9692/11ee9da3_9e07_909a_9ff6_c30ad48afa3c_TB9692_11.jpg) 2 SO3(g)
2 SO3(g)
A) [SO2] = 0.040M, [SO3] = 0.060M
B) [SO2] = 0.080M, [SO3] = 0.080M
C) [SO2] = 0.080M, [SO3] = 0.120M
D) [SO2] = 0.020M, [SO3] = 0.080M
E) none of these
2 SO2(g) + O2(g)
![<strong>A 0.100 M sample of SO<sub>2</sub> reacts with 0.060 M O<sub>2</sub> to produce SO<sub>3</sub> by the reaction shown below. There was no SO<sub>3</sub> present initially. The concentration of O<sub>2</sub> measured at equilibrium is found to be 0.020 M. What are the equilibrium concentrations of SO<sub>2</sub> and SO<sub>3</sub>? 2 SO<sub>2</sub>(g) + O<sub>2</sub>(g) 2 SO<sub>3</sub>(g)</strong> A) [SO<sub>2</sub>] = 0.040M, [SO<sub>3</sub>] = 0.060M B) [SO<sub>2</sub>] = 0.080M, [SO<sub>3</sub>] = 0.080M C) [SO<sub>2</sub>] = 0.080M, [SO<sub>3</sub>] = 0.120M D) [SO<sub>2</sub>] = 0.020M, [SO<sub>3</sub>] = 0.080M E) none of these](https://storage.examlex.com/TB9692/11ee9da3_9e07_909a_9ff6_c30ad48afa3c_TB9692_11.jpg) 2 SO3(g)
2 SO3(g)A) [SO2] = 0.040M, [SO3] = 0.060M
B) [SO2] = 0.080M, [SO3] = 0.080M
C) [SO2] = 0.080M, [SO3] = 0.120M
D) [SO2] = 0.020M, [SO3] = 0.080M
E) none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate the concentrations of Cl2 and ClF3 at equilibrium if a reaction that initially contained 1.000 M concentrations of both Cl2 and F2 is found to have an F2 concentration of 0.700 M at equilibrium. ![<strong>Calculate the concentrations of Cl<sub>2</sub> and ClF<sub>3</sub> at equilibrium if a reaction that initially contained 1.000 M concentrations of both Cl<sub>2</sub> and F<sub>2 </sub>is found to have an F<sub>2</sub> concentration of 0.700 M at equilibrium. </strong> A) [Cl<sub>2</sub>] = 0.700M [ClF<sub>3</sub>] = 0.700M B) [Cl<sub>2</sub>] = 0.700M [ClF<sub>3</sub>] = 0.300M C) [Cl<sub>2</sub>] = 0.500M [ClF<sub>3</sub>] = 0.300M D) [Cl<sub>2</sub>] = 0.900M [ClF<sub>3</sub>] = 0.200M E) none of these](https://storage.examlex.com/TB9692/11ee9da3_9e07_b7ab_9ff6_577740a7a47f_TB9692_00.jpg)
A) [Cl2] = 0.700M [ClF3] = 0.700M
B) [Cl2] = 0.700M [ClF3] = 0.300M
C) [Cl2] = 0.500M [ClF3] = 0.300M
D) [Cl2] = 0.900M [ClF3] = 0.200M
E) none of these
![<strong>Calculate the concentrations of Cl<sub>2</sub> and ClF<sub>3</sub> at equilibrium if a reaction that initially contained 1.000 M concentrations of both Cl<sub>2</sub> and F<sub>2 </sub>is found to have an F<sub>2</sub> concentration of 0.700 M at equilibrium. </strong> A) [Cl<sub>2</sub>] = 0.700M [ClF<sub>3</sub>] = 0.700M B) [Cl<sub>2</sub>] = 0.700M [ClF<sub>3</sub>] = 0.300M C) [Cl<sub>2</sub>] = 0.500M [ClF<sub>3</sub>] = 0.300M D) [Cl<sub>2</sub>] = 0.900M [ClF<sub>3</sub>] = 0.200M E) none of these](https://storage.examlex.com/TB9692/11ee9da3_9e07_b7ab_9ff6_577740a7a47f_TB9692_00.jpg)
A) [Cl2] = 0.700M [ClF3] = 0.700M
B) [Cl2] = 0.700M [ClF3] = 0.300M
C) [Cl2] = 0.500M [ClF3] = 0.300M
D) [Cl2] = 0.900M [ClF3] = 0.200M
E) none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
For the reaction
2 NOCl(g)![<strong>For the reaction 2 NOCl(g) 2 NO(g) + Cl<sub>2</sub> K<sub>c</sub> = 5.6 x 10<sup>-6</sup> (at 400 K) What is the relationship between the magnitude of the changes in the concentrations of NOCl and Cl<sub>2</sub> as this reaction comes to equilibrium?</strong> A) [NOCl] = [Cl<sub>2</sub>] B) [NOCl] = 2 [Cl<sub>2</sub>] C) [Cl<sub>2</sub>] = 2 [NOCl] D) [NOCl] = 1/[Cl<sub>2</sub>] E) 2 [NOCl] = [Cl<sub>2</sub>]](https://storage.examlex.com/TB9692/11ee9da3_9e07_b7ac_9ff6_b7b8155013da_TB9692_11.jpg) 2 NO(g) + Cl2 Kc = 5.6 x 10-6 (at 400 K)
2 NO(g) + Cl2 Kc = 5.6 x 10-6 (at 400 K)
What is the relationship between the magnitude of the changes in the concentrations of NOCl and Cl2 as this reaction comes to equilibrium?
A) [NOCl] = [Cl2]
B) [NOCl] = 2 [Cl2]
C) [Cl2] = 2 [NOCl]
D) [NOCl] = 1/[Cl2]
E) 2 [NOCl] = [Cl2]
2 NOCl(g)
![<strong>For the reaction 2 NOCl(g) 2 NO(g) + Cl<sub>2</sub> K<sub>c</sub> = 5.6 x 10<sup>-6</sup> (at 400 K) What is the relationship between the magnitude of the changes in the concentrations of NOCl and Cl<sub>2</sub> as this reaction comes to equilibrium?</strong> A) [NOCl] = [Cl<sub>2</sub>] B) [NOCl] = 2 [Cl<sub>2</sub>] C) [Cl<sub>2</sub>] = 2 [NOCl] D) [NOCl] = 1/[Cl<sub>2</sub>] E) 2 [NOCl] = [Cl<sub>2</sub>]](https://storage.examlex.com/TB9692/11ee9da3_9e07_b7ac_9ff6_b7b8155013da_TB9692_11.jpg) 2 NO(g) + Cl2 Kc = 5.6 x 10-6 (at 400 K)
2 NO(g) + Cl2 Kc = 5.6 x 10-6 (at 400 K)What is the relationship between the magnitude of the changes in the concentrations of NOCl and Cl2 as this reaction comes to equilibrium?
A) [NOCl] = [Cl2]
B) [NOCl] = 2 [Cl2]
C) [Cl2] = 2 [NOCl]
D) [NOCl] = 1/[Cl2]
E) 2 [NOCl] = [Cl2]

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
What is the relationship between the changes in the concentration of NO2 and O2 as the following reaction comes to equilibrium?
2 NO2(g)![<strong>What is the relationship between the changes in the concentration of NO<sub>2</sub> and O<sub>2</sub> as the following reaction comes to equilibrium? 2 NO<sub>2</sub>(g) 2 NO(g) + O<sub>2</sub>(g) K<sub>c</sub> = 7.4 x 10<sup>-16</sup> (at 25°C)</strong> A) [NO<sub>2</sub>] = -[O<sub>2</sub>] B) [NO<sub>2</sub>] = -2 [O<sub>2</sub>] C) [O<sub>2</sub>] = -2 [NO<sub>2</sub>] D) There is no relationship between the changes in the concentrations of these substances. E) none of the above](https://storage.examlex.com/TB9692/11ee9da3_9e07_b7ad_9ff6_05647ce478ae_TB9692_11.jpg) 2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
A) [NO2] = -[O2]
B) [NO2] = -2 [O2]
C) [O2] = -2 [NO2]
D) There is no relationship between the changes in the concentrations of these substances.
E) none of the above
2 NO2(g)
![<strong>What is the relationship between the changes in the concentration of NO<sub>2</sub> and O<sub>2</sub> as the following reaction comes to equilibrium? 2 NO<sub>2</sub>(g) 2 NO(g) + O<sub>2</sub>(g) K<sub>c</sub> = 7.4 x 10<sup>-16</sup> (at 25°C)</strong> A) [NO<sub>2</sub>] = -[O<sub>2</sub>] B) [NO<sub>2</sub>] = -2 [O<sub>2</sub>] C) [O<sub>2</sub>] = -2 [NO<sub>2</sub>] D) There is no relationship between the changes in the concentrations of these substances. E) none of the above](https://storage.examlex.com/TB9692/11ee9da3_9e07_b7ad_9ff6_05647ce478ae_TB9692_11.jpg) 2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)A) [NO2] = -[O2]
B) [NO2] = -2 [O2]
C) [O2] = -2 [NO2]
D) There is no relationship between the changes in the concentrations of these substances.
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
For the following reaction
2 C2H6(g) + 7 O2(g)![<strong>For the following reaction 2 C<sub>2</sub>H<sub>6</sub>(g) + 7 O<sub>2</sub>(g) 4 CO<sub>2</sub>(g) + 6 H<sub>2</sub>O(g) If the reaction mixture initially contained all species at the same initial concentration, C, and if the concentration of O<sub>2</sub> at equilibrium was expressed as [O<sub>2</sub>] = C + 7 \Delta C , then the concentration of H<sub>2</sub>O at equilibrium would be correctly expressed as [H<sub>2</sub>O] =</strong> A) C + \Delta C B) C - \Delta C C) C + 6 \Delta C D) C - 6 \Delta C E) none of the above](https://storage.examlex.com/TB9692/11ee9da3_9e07_debe_9ff6_b37c2c390dbf_TB9692_11.jpg) 4 CO2(g) + 6 H2O(g)
4 CO2(g) + 6 H2O(g)
If the reaction mixture initially contained all species at the same initial concentration, C, and if the concentration of O2 at equilibrium was expressed as [O2] = C + 7 C , then the concentration of H2O at equilibrium would be correctly expressed as [H2O] =
A) C + C
B) C - C
C) C + 6 C
D) C - 6 C
E) none of the above
2 C2H6(g) + 7 O2(g)
![<strong>For the following reaction 2 C<sub>2</sub>H<sub>6</sub>(g) + 7 O<sub>2</sub>(g) 4 CO<sub>2</sub>(g) + 6 H<sub>2</sub>O(g) If the reaction mixture initially contained all species at the same initial concentration, C, and if the concentration of O<sub>2</sub> at equilibrium was expressed as [O<sub>2</sub>] = C + 7 \Delta C , then the concentration of H<sub>2</sub>O at equilibrium would be correctly expressed as [H<sub>2</sub>O] =</strong> A) C + \Delta C B) C - \Delta C C) C + 6 \Delta C D) C - 6 \Delta C E) none of the above](https://storage.examlex.com/TB9692/11ee9da3_9e07_debe_9ff6_b37c2c390dbf_TB9692_11.jpg) 4 CO2(g) + 6 H2O(g)
4 CO2(g) + 6 H2O(g)If the reaction mixture initially contained all species at the same initial concentration, C, and if the concentration of O2 at equilibrium was expressed as [O2] = C + 7 C , then the concentration of H2O at equilibrium would be correctly expressed as [H2O] =
A) C + C
B) C - C
C) C + 6 C
D) C - 6 C
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the COCl2, CO, and Cl2 concentrations when the following gas-phase reaction reaches equilibrium at 300°C.
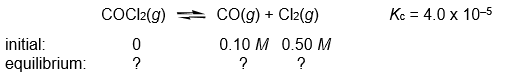
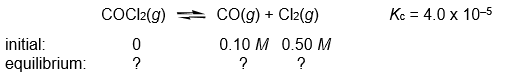

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate the NO, NO2, and O2 concentrations in the following gas-phase reaction at equilibrium at 200°C.
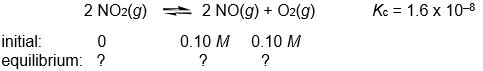
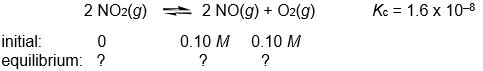

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the equilibrium concentrations of SO3, SO2, and O2 if 0.100 moles of SO3 in a 10-L flask decompose to form SO2 and O2 at 500 K.
2 SO3(g)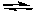 2 SO2(g) + O2(g) Kc = 1.4 x 10-11
2 SO2(g) + O2(g) Kc = 1.4 x 10-11
2 SO3(g)
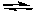 2 SO2(g) + O2(g) Kc = 1.4 x 10-11
2 SO2(g) + O2(g) Kc = 1.4 x 10-11
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the concentrations of all reactants and products when a mixture
that is initially 0.10 M in N2, H2, and NH3 comes to equilibrium at 600 K.
N2(g) + 3 H2(g)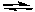 2 NH3(g) Kc = 0.045
2 NH3(g) Kc = 0.045
that is initially 0.10 M in N2, H2, and NH3 comes to equilibrium at 600 K.
N2(g) + 3 H2(g)
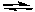 2 NH3(g) Kc = 0.045
2 NH3(g) Kc = 0.045
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
Assume an equilibrium constant Kc = 1.7 x 10-2 for the reaction:
N2(g) + 3 H2(g)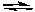 2 NH3(g)
2 NH3(g)
Calculate (to a first approximation) the equilibrium concentrations of NH3, H2 and N2 when 0.10 moles per liter each of N2 and H2 are mixed and allowed to react to equilibrium.
N2(g) + 3 H2(g)
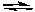 2 NH3(g)
2 NH3(g)Calculate (to a first approximation) the equilibrium concentrations of NH3, H2 and N2 when 0.10 moles per liter each of N2 and H2 are mixed and allowed to react to equilibrium.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate the COCl2, CO, and Cl2 concentrations when the following gas-phase reaction reaches equilibrium at 300°C.
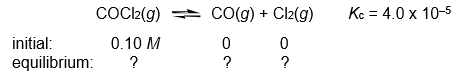
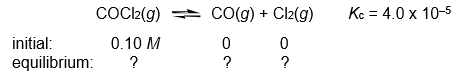

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
Calculate the NO, NO2, and O2 concentrations in the following gas-phase reaction at equilibrium at 200°C.
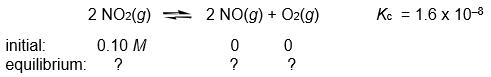
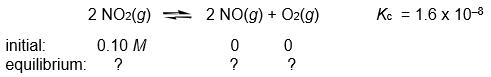

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 2H2(g) + O2(g)
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-Starting with 0.10 M H2O(g), what would be the best strategy for solving for the equilibrium concentrations of H2O, H2 and O2?
A) Since the change in the concentrations are expected to be large going to equilibrium, the equation will have to be solved exactly.
B) Since the changes in the concentrations are expected to be large going to equilibrium, it is best to assume that all of the H2O(g) is converted to products and then calculate the equilibrium concentration.
C) Since the changes in concentrations are expected to be small going to equilibrium, it is best to assume that the changes will be small relative to the initial concentration of H2O(g).
D) Since the changes in concentrations are expected to be small going to equilibrium, it is best to assume that the changes will be large relative to the initial concentration of H2O(g).
E) There is no reasonable way to even approximate the answer to this problem.
based on the following reaction:
2H2O(g)
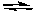 2H2(g) + O2(g)
2H2(g) + O2(g)Kc = 2 x 10-42 (at 25oC)
-Starting with 0.10 M H2O(g), what would be the best strategy for solving for the equilibrium concentrations of H2O, H2 and O2?
A) Since the change in the concentrations are expected to be large going to equilibrium, the equation will have to be solved exactly.
B) Since the changes in the concentrations are expected to be large going to equilibrium, it is best to assume that all of the H2O(g) is converted to products and then calculate the equilibrium concentration.
C) Since the changes in concentrations are expected to be small going to equilibrium, it is best to assume that the changes will be small relative to the initial concentration of H2O(g).
D) Since the changes in concentrations are expected to be small going to equilibrium, it is best to assume that the changes will be large relative to the initial concentration of H2O(g).
E) There is no reasonable way to even approximate the answer to this problem.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g)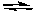 2H2(g) + O2(g)
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-Starting with only 0.10 M H2O(g), which equation correctly shows the equilibrium concentrations of the reactants and products? ( C represents the change in concentration of O2(g))
A)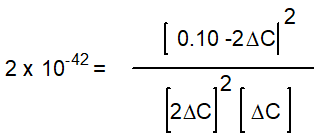
B)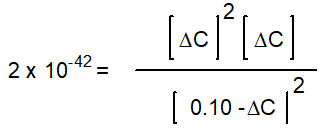
C)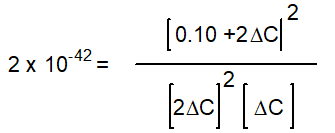
D)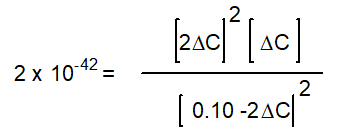
E) None of the above
based on the following reaction:
2H2O(g)
 2H2(g) + O2(g)
2H2(g) + O2(g)Kc = 2 x 10-42 (at 25oC)
-Starting with only 0.10 M H2O(g), which equation correctly shows the equilibrium concentrations of the reactants and products? ( C represents the change in concentration of O2(g))
A)
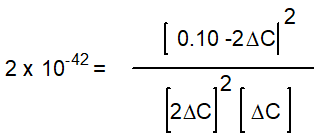
B)
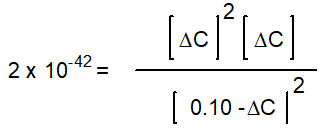
C)
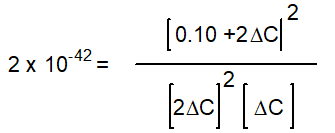
D)
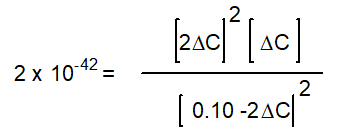
E) None of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 2H2(g) + O2(g)
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-The equation below solves for the change in concentration for the given reaction assuming the initial concentration of H2O(g) is 0.10 M. Which equation will result if it is assumed that the change will be much smaller than the initial concentration of H2O(g)?
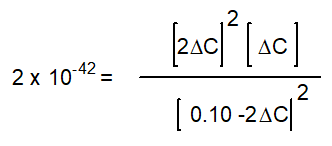
A)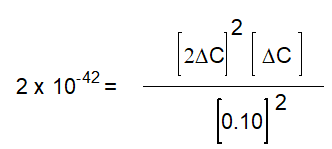
B)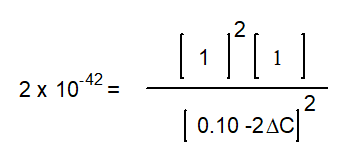
C)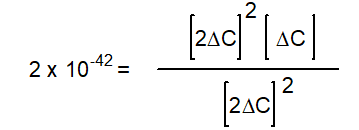
D)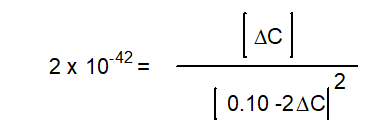
E) None of the above
based on the following reaction:
2H2O(g)
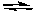 2H2(g) + O2(g)
2H2(g) + O2(g)Kc = 2 x 10-42 (at 25oC)
-The equation below solves for the change in concentration for the given reaction assuming the initial concentration of H2O(g) is 0.10 M. Which equation will result if it is assumed that the change will be much smaller than the initial concentration of H2O(g)?
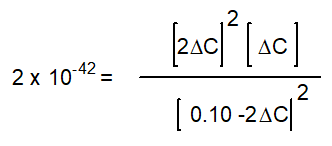
A)
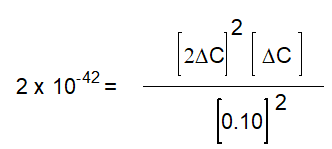
B)
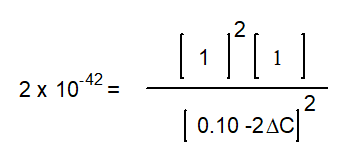
C)
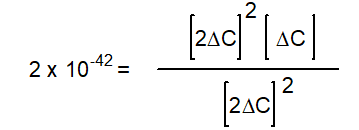
D)
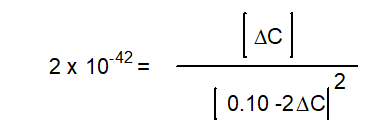
E) None of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 2H2(g) + O2(g)
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-When solving a problem with the initial conditions outlined below, we rearrange the problem to give an intermediate set of conditions where the concentration of one of the reactants or products is equal to zero.
Fe(CO)5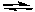 Fe(CO)4 + CO
Fe(CO)4 + CO
Initial: 0.10 M 0.10 M 0.005 M
Our choice of whether to push the reaction all the way to the right or all the way to the left is determined by which of the following goals?
A) to make both Qc and Kc large
B) to make both Qc and Kc small
C) to bring Qc as close as possible to Kc
D) to make the difference between Qc and Kc as large as possible
based on the following reaction:
2H2O(g)
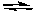 2H2(g) + O2(g)
2H2(g) + O2(g)Kc = 2 x 10-42 (at 25oC)
-When solving a problem with the initial conditions outlined below, we rearrange the problem to give an intermediate set of conditions where the concentration of one of the reactants or products is equal to zero.
Fe(CO)5
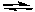 Fe(CO)4 + CO
Fe(CO)4 + COInitial: 0.10 M 0.10 M 0.005 M
Our choice of whether to push the reaction all the way to the right or all the way to the left is determined by which of the following goals?
A) to make both Qc and Kc large
B) to make both Qc and Kc small
C) to bring Qc as close as possible to Kc
D) to make the difference between Qc and Kc as large as possible

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
What would happen if F2 was removed from the following reaction while it was at equilibrium?
Cl2(g) + 3 F2(g)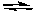 2 ClF3(g)
2 ClF3(g)
A) The concentration of Cl2 would increase as the reaction returned to equilibrium.
B) The concentration of both Cl2 and F2 would increase as the reaction returned to equilibrium.
C) The concentration of Cl2 would decrease as the reaction returned to equilibrium.
D) The concentration of both Cl2 and F2 would decrease as the reaction returned to equilibrium.
E) The concentration of both F2 and Cl2 would remain the same because the reaction would be at equilibrium.
Cl2(g) + 3 F2(g)
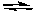 2 ClF3(g)
2 ClF3(g)A) The concentration of Cl2 would increase as the reaction returned to equilibrium.
B) The concentration of both Cl2 and F2 would increase as the reaction returned to equilibrium.
C) The concentration of Cl2 would decrease as the reaction returned to equilibrium.
D) The concentration of both Cl2 and F2 would decrease as the reaction returned to equilibrium.
E) The concentration of both F2 and Cl2 would remain the same because the reaction would be at equilibrium.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
What is the effect of increasing the temperature on the following exothermic reaction?
2 CO(g) + O2(g)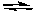 2 CO2(g)
2 CO2(g)
A) The equilibrium will shift to the right.
B) The equilibrium will shift to the left.
C) The partial pressure of CO2 will increase.
D) The partial pressure of O2 will decrease.
E) It will have no effect.
2 CO(g) + O2(g)
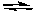 2 CO2(g)
2 CO2(g)A) The equilibrium will shift to the right.
B) The equilibrium will shift to the left.
C) The partial pressure of CO2 will increase.
D) The partial pressure of O2 will decrease.
E) It will have no effect.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
What would happen to the extent of the decomposition of PCl5 at a given temperature if the pressure on the system were decreased by increasing the volume of the container at a constant temperature?
PCl5(g)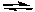 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
A) It would increase.
B) It would decrease.
C) It would remain constant.
D) Not enough information is given to determine this.
PCl5(g)
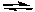 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)A) It would increase.
B) It would decrease.
C) It would remain constant.
D) Not enough information is given to determine this.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following factors will cause this chemical reaction to shift its equilibrium to the right?
Xe(g) + 2 F2(g)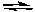 XeF4(g) H = -218 kJ/molrxn
XeF4(g) H = -218 kJ/molrxn
A) increasing the temperature
B) increasing the pressure
C) decreasing the pressure
D) increasing the concentration of XeF4
E) decreasing the concentration of F2
Xe(g) + 2 F2(g)
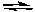 XeF4(g) H = -218 kJ/molrxn
XeF4(g) H = -218 kJ/molrxnA) increasing the temperature
B) increasing the pressure
C) decreasing the pressure
D) increasing the concentration of XeF4
E) decreasing the concentration of F2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
The following chemical reaction has reached equilibrium. Which of the changes listed below would cause the equilibrium to shift back toward the reactants?
N2(g) + 3 H2(g)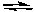 2 NH3(g) H = -92.2 kJ/molrxn
2 NH3(g) H = -92.2 kJ/molrxn
A) increasing the pressure
B) increasing the concentration of N2
C) increasing the temperature
D) decreasing the concentration of NH3
E) none of these
N2(g) + 3 H2(g)
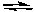 2 NH3(g) H = -92.2 kJ/molrxn
2 NH3(g) H = -92.2 kJ/molrxnA) increasing the pressure
B) increasing the concentration of N2
C) increasing the temperature
D) decreasing the concentration of NH3
E) none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
The following reaction is at equilibrium. Which of the conditions listed below will cause the reaction to shift toward the products?
2 SO3(g) + 2 Cl2(g)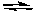 2 SO2Cl2(g)+ O2(g)
2 SO2Cl2(g)+ O2(g)
A) The concentration of O2 is increased.
B) The concentration of SO2Cl2 is decreased.
C) The concentration of SO3 is decreased.
D) The pressure is decreased.
E) none of these
2 SO3(g) + 2 Cl2(g)
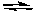 2 SO2Cl2(g)+ O2(g)
2 SO2Cl2(g)+ O2(g)A) The concentration of O2 is increased.
B) The concentration of SO2Cl2 is decreased.
C) The concentration of SO3 is decreased.
D) The pressure is decreased.
E) none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
The following reactions are at equilibrium. Beside each reaction is a change that is made to the system which may cause a shift in equilibrium. Which of the reactions will shift its equilibrium toward the products?
A) N2(g) + O2(g)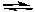 2 NO(g) the concentration of NO is increased
2 NO(g) the concentration of NO is increased
B) PF5(g)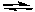 PF3(g) + F2(g) the concentration of F2 is increased
PF3(g) + F2(g) the concentration of F2 is increased
C) Cl2(g) + 3 F2(g)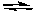 2 ClF3(g) the concentration of Cl2 is decreased
2 ClF3(g) the concentration of Cl2 is decreased
D) 2 SO3(g)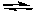 2 SO2(g) + O2(g) the concentration of SO2 is decreased
2 SO2(g) + O2(g) the concentration of SO2 is decreased
E) none of these
A) N2(g) + O2(g)
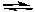 2 NO(g) the concentration of NO is increased
2 NO(g) the concentration of NO is increasedB) PF5(g)
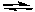 PF3(g) + F2(g) the concentration of F2 is increased
PF3(g) + F2(g) the concentration of F2 is increasedC) Cl2(g) + 3 F2(g)
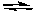 2 ClF3(g) the concentration of Cl2 is decreased
2 ClF3(g) the concentration of Cl2 is decreasedD) 2 SO3(g)
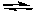 2 SO2(g) + O2(g) the concentration of SO2 is decreased
2 SO2(g) + O2(g) the concentration of SO2 is decreasedE) none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
The following reactions are at equilibrium. Which of the reactions will shift its equilibrium toward the reactant side if the pressure is increased while holding the temperature constant?
A) 2 NO(g) + 2 H2(g)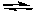 N2(g) + 2 H2O(g)
N2(g) + 2 H2O(g)
B) 2 NOCl(g)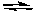 2 NO(g) + Cl2(g)
2 NO(g) + Cl2(g)
C) 2 NO(g) + O2(g)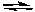 2 NO2(g)
2 NO2(g)
D) N2(g) + O2(g)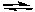 2 NO(g)
2 NO(g)
E) NO(g) + NO2(g)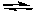 N2O3(g)
N2O3(g)
A) 2 NO(g) + 2 H2(g)
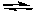 N2(g) + 2 H2O(g)
N2(g) + 2 H2O(g)B) 2 NOCl(g)
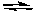 2 NO(g) + Cl2(g)
2 NO(g) + Cl2(g)C) 2 NO(g) + O2(g)
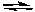 2 NO2(g)
2 NO2(g)D) N2(g) + O2(g)
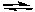 2 NO(g)
2 NO(g)E) NO(g) + NO2(g)
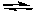 N2O3(g)
N2O3(g)
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
What would happen if Cl2 is added to the following system at equilibrium?
H2(g) + Cl2(g)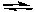 2 HCl(g)
2 HCl(g)
A) The concentrations of both H2 and HCl would remain the same.
B) The concentrations of both H2 and HCl would increase.
C) The concentrations of both H2 and HCl would decrease.
D) The concentration of H2 would increase, but HCl would decrease.
E) The concentration of H2 would decrease, but HCl would increase.
H2(g) + Cl2(g)
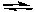 2 HCl(g)
2 HCl(g)A) The concentrations of both H2 and HCl would remain the same.
B) The concentrations of both H2 and HCl would increase.
C) The concentrations of both H2 and HCl would decrease.
D) The concentration of H2 would increase, but HCl would decrease.
E) The concentration of H2 would decrease, but HCl would increase.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following statements correctly describes the following reaction?
2 NH3(g)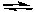 N2(g) + 3 H2(g) H = 92.2 kJ
N2(g) + 3 H2(g) H = 92.2 kJ
A) An increase in either pressure or temperature shifts the reaction to the left.
B) If the pressure of N2 is doubled and a new equilibrium established, the concentration of H2 at equilibrium will be reduced.
C) More ammonia will be present in the equilibrium mixture if a catalyst is added.
D) none of the above
E) all of the above
2 NH3(g)
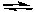 N2(g) + 3 H2(g) H = 92.2 kJ
N2(g) + 3 H2(g) H = 92.2 kJA) An increase in either pressure or temperature shifts the reaction to the left.
B) If the pressure of N2 is doubled and a new equilibrium established, the concentration of H2 at equilibrium will be reduced.
C) More ammonia will be present in the equilibrium mixture if a catalyst is added.
D) none of the above
E) all of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
What would be the effect of removing some NO2 from the following system after the reaction reaches equilibrium at 25°C?
2 NO2(g)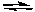 2 NO(g) + O2(g) Kc = 7.4 x 10-16
2 NO(g) + O2(g) Kc = 7.4 x 10-16
A) The NO and O2 concentrations would increase.
B) The NO and O2 concentrations would decrease.
C) The NO concentration would increase but O2 would decrease.
D) The O2 concentration would increase but NO would decrease.
E) The concentrations of NO and O2 would remain constant.
2 NO2(g)
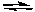 2 NO(g) + O2(g) Kc = 7.4 x 10-16
2 NO(g) + O2(g) Kc = 7.4 x 10-16A) The NO and O2 concentrations would increase.
B) The NO and O2 concentrations would decrease.
C) The NO concentration would increase but O2 would decrease.
D) The O2 concentration would increase but NO would decrease.
E) The concentrations of NO and O2 would remain constant.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
The addition of I2(g) to a fixed volume container with I2(g), H2(g), and HI(g) at equilibrium at a given temperature would cause
H2(g) + I2(g)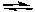 2 HI(g)
2 HI(g)
A) the concentrations of both H2 and HI to decrease.
B) the concentrations of both H2 and HI to increase.
C) the concentration of H2 to increase and HI to decrease.
D) the concentration of H2 to decrease and HI to increase.
E) Not enough information is given to determine how the concentrations will change.
H2(g) + I2(g)
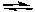 2 HI(g)
2 HI(g)A) the concentrations of both H2 and HI to decrease.
B) the concentrations of both H2 and HI to increase.
C) the concentration of H2 to increase and HI to decrease.
D) the concentration of H2 to decrease and HI to increase.
E) Not enough information is given to determine how the concentrations will change.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
What would happen if O2 were removed from the following system at equilibrium at 25°C?
2 NO2(g)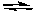 2 NO(g) + O2(g) Kc = 7.4 x 10-1 at 25oC
2 NO(g) + O2(g) Kc = 7.4 x 10-1 at 25oC
A) The NO2 and NO concentrations would increase.
B) The NO2 and NO concentrations would decrease.
C) The NO2 concentration would increase and the NO concentration would decrease.
D) The NO2 concentration would decrease and the NO concentration would increase.
E) none of the above
2 NO2(g)
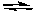 2 NO(g) + O2(g) Kc = 7.4 x 10-1 at 25oC
2 NO(g) + O2(g) Kc = 7.4 x 10-1 at 25oCA) The NO2 and NO concentrations would increase.
B) The NO2 and NO concentrations would decrease.
C) The NO2 concentration would increase and the NO concentration would decrease.
D) The NO2 concentration would decrease and the NO concentration would increase.
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
The equilibrium constant for the following reaction becomes larger as the temperature at which the reaction is run increases.
2 NOCl(g)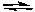 2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 at 400 K
2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 at 400 K
What does this tell us about the reaction?
A) Heat is given off in the reaction.
B) Heat is absorbed in the reaction.
C) Heat is neither given off nor absorbed in the reaction.
D) Increasing the amount of NOCl would also increase the equilibrium constant for the reaction.
E) Increasing the amount of NO would also increase the equilibrium constant for the reaction.
2 NOCl(g)
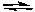 2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 at 400 K
2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 at 400 KWhat does this tell us about the reaction?
A) Heat is given off in the reaction.
B) Heat is absorbed in the reaction.
C) Heat is neither given off nor absorbed in the reaction.
D) Increasing the amount of NOCl would also increase the equilibrium constant for the reaction.
E) Increasing the amount of NO would also increase the equilibrium constant for the reaction.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
What is the effect of increasing the temperature on the following reaction?
2 CO(g) + O2(g)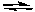 2 CO2(g) + heat
2 CO2(g) + heat
A) The equilibrium will shift to the left.
B) The equilibrium will shift to the right.
C) The partial pressure of CO2 will increase.
D) The partial pressure of O2 will decrease.
E) It will have no effect.
2 CO(g) + O2(g)
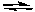 2 CO2(g) + heat
2 CO2(g) + heatA) The equilibrium will shift to the left.
B) The equilibrium will shift to the right.
C) The partial pressure of CO2 will increase.
D) The partial pressure of O2 will decrease.
E) It will have no effect.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
What would happen to the extent of the decomposition of PCl5 at a given temperature if the pressure on the system were decreased?
PCl5(g)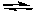 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
A) It would increase.
B) It would decrease.
C) It would remain constant.
D) Not enough information is given to determine this.
PCl5(g)
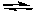 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)A) It would increase.
B) It would decrease.
C) It would remain constant.
D) Not enough information is given to determine this.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
What would be the effect of decreasing the pressure on the following reaction after it reaches equilibrium?
2 NO2(g)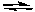 2 NO(g) + O2(g)
2 NO(g) + O2(g)
A) The NO and O2 concentrations would increase.
B) The NO and O2 concentrations would decrease.
C) The NO concentration would increase but O2 would decrease.
D) The O2 concentration would increase but NO would decrease.
E) The concentrations of NO and O2 would remain constant.
2 NO2(g)
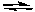 2 NO(g) + O2(g)
2 NO(g) + O2(g)A) The NO and O2 concentrations would increase.
B) The NO and O2 concentrations would decrease.
C) The NO concentration would increase but O2 would decrease.
D) The O2 concentration would increase but NO would decrease.
E) The concentrations of NO and O2 would remain constant.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
One of the products of the reaction between NO2 and CO is CO2. By writing the equation for the reaction (Hint: the coefficients for all reactants and products are 1), decide whether an increase in pressure:
A) favors CO2 formation
B) decreases CO2 formation
C) has no effect on CO2 formation
D) cannot tell from information supplied
A) favors CO2 formation
B) decreases CO2 formation
C) has no effect on CO2 formation
D) cannot tell from information supplied

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following equilibria would not be affected by changes in pressure?
A) 2 NO(g) + O2(g)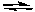 2 NO2(g)
2 NO2(g)
B) N2O4(g)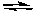 2 NO2(g)
2 NO2(g)
C) 4 NH3(g) + 5 O2(g)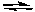 4 NO(g) + 6 H2O(g)
4 NO(g) + 6 H2O(g)
D) CaCO3(s)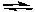 CaO(s) + CO2(g)
CaO(s) + CO2(g)
E) CO(g) + H2O(g)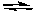 CO2(g) + H2(g)
CO2(g) + H2(g)
A) 2 NO(g) + O2(g)
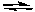 2 NO2(g)
2 NO2(g)B) N2O4(g)
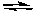 2 NO2(g)
2 NO2(g)C) 4 NH3(g) + 5 O2(g)
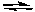 4 NO(g) + 6 H2O(g)
4 NO(g) + 6 H2O(g)D) CaCO3(s)
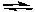 CaO(s) + CO2(g)
CaO(s) + CO2(g)E) CO(g) + H2O(g)
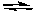 CO2(g) + H2(g)
CO2(g) + H2(g)
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
What is the concentration in moles per liter of pure PbSO4(s) given that it has a density of 6.29 g/cm3?
A) 6.29
B) 20.7
C) 1.00
D) 303
E) The concentration of a pure solid cannot be found.
A) 6.29
B) 20.7
C) 1.00
D) 303
E) The concentration of a pure solid cannot be found.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
What is the correct solubility constant expression for PbSO4?
A)![<strong>What is the correct solubility constant expression for PbSO<sub>4</sub>?</strong> A) B) K<sub>sp</sub> = <sup> </sup>[Pb<sup>2+</sup>][SO<sub>2</sub><sup>2-</sup>][O<sub>2</sub>]<sup> </sup> C) K<sub>sp</sub> = <sup> </sup>[Pb<sup>2+</sup>][SO<sub>4</sub><sup>2-</sup>] D) E) none of the above](https://storage.examlex.com/TB9692/11efc1e2_8406_c3d0_9fac_8b6309466f4b_TB9692_00.jpg)
B) Ksp = [Pb2+][SO22-][O2]
C) Ksp = [Pb2+][SO42-]
D)![<strong>What is the correct solubility constant expression for PbSO<sub>4</sub>?</strong> A) B) K<sub>sp</sub> = <sup> </sup>[Pb<sup>2+</sup>][SO<sub>2</sub><sup>2-</sup>][O<sub>2</sub>]<sup> </sup> C) K<sub>sp</sub> = <sup> </sup>[Pb<sup>2+</sup>][SO<sub>4</sub><sup>2-</sup>] D) E) none of the above](https://storage.examlex.com/TB9692/11efc1e2_960b_5821_9fac_8148146b5908_TB9692_00.jpg)
E) none of the above
A)
![<strong>What is the correct solubility constant expression for PbSO<sub>4</sub>?</strong> A) B) K<sub>sp</sub> = <sup> </sup>[Pb<sup>2+</sup>][SO<sub>2</sub><sup>2-</sup>][O<sub>2</sub>]<sup> </sup> C) K<sub>sp</sub> = <sup> </sup>[Pb<sup>2+</sup>][SO<sub>4</sub><sup>2-</sup>] D) E) none of the above](https://storage.examlex.com/TB9692/11efc1e2_8406_c3d0_9fac_8b6309466f4b_TB9692_00.jpg)
B) Ksp = [Pb2+][SO22-][O2]
C) Ksp = [Pb2+][SO42-]
D)
![<strong>What is the correct solubility constant expression for PbSO<sub>4</sub>?</strong> A) B) K<sub>sp</sub> = <sup> </sup>[Pb<sup>2+</sup>][SO<sub>2</sub><sup>2-</sup>][O<sub>2</sub>]<sup> </sup> C) K<sub>sp</sub> = <sup> </sup>[Pb<sup>2+</sup>][SO<sub>4</sub><sup>2-</sup>] D) E) none of the above](https://storage.examlex.com/TB9692/11efc1e2_960b_5821_9fac_8148146b5908_TB9692_00.jpg)
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
Which is the correct reaction for the solubility constant expression (Ksp) of CaF2(s)?
A) 2F-(aq) + Ca2+(aq)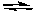 CaF2(s)
CaF2(s)
B) 2F(aq) + Ca (aq)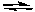 CaF2(s)
CaF2(s)
C) CaF2(s)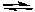 CaF2(g)
CaF2(g)
D) CaF2(s)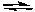 Ca(g) + 2F(g)
Ca(g) + 2F(g)
E) CaF2(s)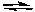 Ca2+(aq) + 2F-(aq)
Ca2+(aq) + 2F-(aq)
A) 2F-(aq) + Ca2+(aq)
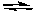 CaF2(s)
CaF2(s)B) 2F(aq) + Ca (aq)
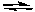 CaF2(s)
CaF2(s)C) CaF2(s)
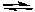 CaF2(g)
CaF2(g)D) CaF2(s)
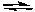 Ca(g) + 2F(g)
Ca(g) + 2F(g)E) CaF2(s)
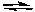 Ca2+(aq) + 2F-(aq)
Ca2+(aq) + 2F-(aq)
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
What is the correct solubility constant expression of Ba3(PO4)2?
A)![<strong>What is the correct solubility constant expression of Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>? </strong> A) B) K<sub>sp</sub> = <sup> </sup>[Ba<sup>2+</sup>]<sup>2</sup>[PO<sub>4</sub><sup>3</sup> <sup>-</sup>]<sup>3 </sup> C) K<sub>sp</sub> = <sup> </sup>[Ba<sup>2+</sup>][PO<sub>4</sub><sup>3</sup> <sup>-</sup>] D) K<sub>sp</sub> = <sup> </sup>[Ba<sup>2+</sup>]<sup>3</sup>[PO<sub>4</sub><sup>3</sup> ]<sup>2</sup> E) None of the above](https://storage.examlex.com/TB9692/11efc1e2_a555_ae72_9fac_897ca8f1fdc3_TB9692_00.jpg)
B) Ksp = [Ba2+]2[PO43 -]3
C) Ksp = [Ba2+][PO43 -]
D) Ksp = [Ba2+]3[PO43 ]2
E) None of the above
A)
![<strong>What is the correct solubility constant expression of Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>? </strong> A) B) K<sub>sp</sub> = <sup> </sup>[Ba<sup>2+</sup>]<sup>2</sup>[PO<sub>4</sub><sup>3</sup> <sup>-</sup>]<sup>3 </sup> C) K<sub>sp</sub> = <sup> </sup>[Ba<sup>2+</sup>][PO<sub>4</sub><sup>3</sup> <sup>-</sup>] D) K<sub>sp</sub> = <sup> </sup>[Ba<sup>2+</sup>]<sup>3</sup>[PO<sub>4</sub><sup>3</sup> ]<sup>2</sup> E) None of the above](https://storage.examlex.com/TB9692/11efc1e2_a555_ae72_9fac_897ca8f1fdc3_TB9692_00.jpg)
B) Ksp = [Ba2+]2[PO43 -]3
C) Ksp = [Ba2+][PO43 -]
D) Ksp = [Ba2+]3[PO43 ]2
E) None of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
What is the correct solubility constant expression for CaF2(s)?
A)![<strong>What is the correct solubility constant expression for CaF<sub>2</sub>(s)?</strong> A) B) K<sub>sp</sub> = <sup> </sup>[Ca<sup>2+</sup>]<sup>2</sup>[ <sup>-</sup> ]<sup> </sup> C) K<sub>sp</sub> = <sup> </sup>[Ca<sup>2+</sup>][ <sup>-</sup> ] D) K<sub>sp</sub> = <sup> </sup>[Ca<sup>2+</sup>][F<sub>2</sub>]<sup>2</sup> E) none of the above](https://storage.examlex.com/TB9692/11efc1e2_b795_c523_9fac_95a7af39905b_TB9692_00.jpg)
B) Ksp = [Ca2+]2[ - ]
C) Ksp = [Ca2+][ - ]
D) Ksp = [Ca2+][F2]2
E) none of the above
A)
![<strong>What is the correct solubility constant expression for CaF<sub>2</sub>(s)?</strong> A) B) K<sub>sp</sub> = <sup> </sup>[Ca<sup>2+</sup>]<sup>2</sup>[ <sup>-</sup> ]<sup> </sup> C) K<sub>sp</sub> = <sup> </sup>[Ca<sup>2+</sup>][ <sup>-</sup> ] D) K<sub>sp</sub> = <sup> </sup>[Ca<sup>2+</sup>][F<sub>2</sub>]<sup>2</sup> E) none of the above](https://storage.examlex.com/TB9692/11efc1e2_b795_c523_9fac_95a7af39905b_TB9692_00.jpg)
B) Ksp = [Ca2+]2[ - ]
C) Ksp = [Ca2+][ - ]
D) Ksp = [Ca2+][F2]2
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
What is the correct solubility constant expression for AgCl(s)?
A) Ksp = [Ag+][Cl - ]
B) Ksp = [Ag+] + [Cl - ]
C) Ksp = [Ag+]/[Cl - ]
D) Ksp = [Ag][Cl]
E) none of the above
A) Ksp = [Ag+][Cl - ]
B) Ksp = [Ag+] + [Cl - ]
C) Ksp = [Ag+]/[Cl - ]
D) Ksp = [Ag][Cl]
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
What is the solubility in moles per liter of AgBr in water given that the Ksp is 5.0 x 10-13?
A) 5.0 x 10 - 13
B) 2.5 x 10 - 13
C) 7.1 x 10 - 7
D) 4.0 x 10 - 6
E) 2.5 x 10 - 7
A) 5.0 x 10 - 13
B) 2.5 x 10 - 13
C) 7.1 x 10 - 7
D) 4.0 x 10 - 6
E) 2.5 x 10 - 7

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
What is the solubility in moles/L of CaF2 in water given that the Ksp is 4.0 x 10 - 11?
A) 2.2 x 10 - 4
B) 4.0 x 10 - 11
C) 3.5 x 10 - 4
D) 6.0 x 10 - 5
E) 2.0 x 10 - 11
A) 2.2 x 10 - 4
B) 4.0 x 10 - 11
C) 3.5 x 10 - 4
D) 6.0 x 10 - 5
E) 2.0 x 10 - 11

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
If 1.3 x 10 - 5 moles of AgCl will dissolve in 1.0 liter of water at 25-C, what is the Ksp of AgCl?
A) 5.1 x 10 - 9
B) 3.6 x 10 - 3
C) 6.4 x 10 - 5
D) 1.3 x 10 - 5
E) 1.7 x 10-10
A) 5.1 x 10 - 9
B) 3.6 x 10 - 3
C) 6.4 x 10 - 5
D) 1.3 x 10 - 5
E) 1.7 x 10-10

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
If 6.5 x 10 - 5 moles of Ag2CrO4 will dissolve in 1.0 liter of water at 25  C, what is the Ksp of Ag2CrO4?
C, what is the Ksp of Ag2CrO4?
A) 6.5 x 10 - 5
B) 4.2 x 10 - 9
C) 2.7 x 10 - 13
D) 1.1 x 10 - 12
E) 3.3 x 10 - 5
 C, what is the Ksp of Ag2CrO4?
C, what is the Ksp of Ag2CrO4?A) 6.5 x 10 - 5
B) 4.2 x 10 - 9
C) 2.7 x 10 - 13
D) 1.1 x 10 - 12
E) 3.3 x 10 - 5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
If the Ksp of PbBr2 is 8.9 x 10-6, what is the solubility of PbBr2(s) in moles/L?
A) 8.9 x 10 - 6
B) 1.3 x 10 -2
C) 3.3 x 10 - 3
D) 0.21
E) none of the above
A) 8.9 x 10 - 6
B) 1.3 x 10 -2
C) 3.3 x 10 - 3
D) 0.21
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
What is the fluoride ion concentration in a saturated solution of SrF2 if the strontium ion concentration is 5.8 x 10 - 4 M?
A) 5.8 x 10 - 4
B) 2.9 x 10 - 4
C) 1.2 x 10 - 3
D) 1.8 x 10 - 3
E) none of the above
A) 5.8 x 10 - 4
B) 2.9 x 10 - 4
C) 1.2 x 10 - 3
D) 1.8 x 10 - 3
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
What is the Ksp of SrF2(s) if 0.073 g of SrF2 dissolves in 1.00 liter of solution?
A) 5.8 x 10 - 4
B) 1.7 x 10 - 3
C) 1.6 x 10 - 3
D) 7.8 x 10 - 10
E) none of the above
A) 5.8 x 10 - 4
B) 1.7 x 10 - 3
C) 1.6 x 10 - 3
D) 7.8 x 10 - 10
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
What is the Ksp of PbBr2 if the concentration of PbBr2(aq) in a saturated solution is 1.3 x 10-2 moles/L?
A) 8.8 x 10 - 6
B) 1.3 x 10 - 2
C) 3.9 x 10 - 2
D) 2.2 x 10 - 2
E) none of the above
A) 8.8 x 10 - 6
B) 1.3 x 10 - 2
C) 3.9 x 10 - 2
D) 2.2 x 10 - 2
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following equations correctly describes the relationship between the concentrations of Pb2+ and Br- ions in a saturated solution of PbBr2(s)?
A) [Pb2+] = 2[Br-]
B) 2[Pb2+] = [Br-]
C) [Pb2+] = [Br-]
D) 3[Pb2+] = [Br-]
E) none of the above
A) [Pb2+] = 2[Br-]
B) 2[Pb2+] = [Br-]
C) [Pb2+] = [Br-]
D) 3[Pb2+] = [Br-]
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following equations correctly describes the relationship between the concentrations of Sr2+ and PO43- ions in a saturated solution of Sr3(PO4)2(s)?
A) 3[Sr2+] = 2[PO43 - ]
B) [Sr2+] = [PO43 - ]
C) 2[Sr2+] = 3[PO43 - ]
D) [Sr2+] = 2/3[PO43 - ]
E) none of the above
A) 3[Sr2+] = 2[PO43 - ]
B) [Sr2+] = [PO43 - ]
C) 2[Sr2+] = 3[PO43 - ]
D) [Sr2+] = 2/3[PO43 - ]
E) none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck


