Exam 10: An Introduction to Kinetics and Equilibrium
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
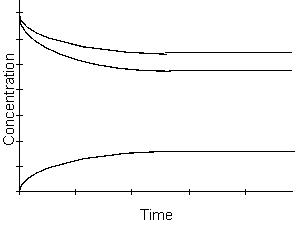 -From the graph above, which of the following is true when equilibrium has been reached?
-From the graph above, which of the following is true when equilibrium has been reached?
Free
(Multiple Choice)
5.0/5  (32)
(32)
Correct Answer:
A
For the following reaction 2 NO2(g) 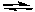 2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
If (NO2) = 0.10 M, (NO) = 0.010 M, and (O2) = 1.0 x 10-5 M, we can correctly predict
2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
If (NO2) = 0.10 M, (NO) = 0.010 M, and (O2) = 1.0 x 10-5 M, we can correctly predict
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
B
What would be the effect of removing some NO2 from the following system after the reaction reaches equilibrium at 25°C?
2 NO2(g) 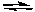 2 NO(g) + O2(g) Kc = 7.4 x 10-16
2 NO(g) + O2(g) Kc = 7.4 x 10-16
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
B
Calculate the NO, NO2, and O2 concentrations in the following gas-phase reaction at equilibrium at 200°C.
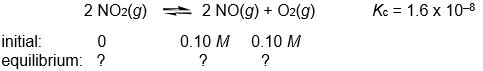
(Essay)
4.9/5  (40)
(40)
For the following reaction:
2 NOCl(g) 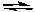 2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO) = 0.989
M at some moment in time, we can conclude that
2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO) = 0.989
M at some moment in time, we can conclude that
(Multiple Choice)
4.9/5  (46)
(46)
What is the correct solubility constant expression for CaF2(s)?
(Multiple Choice)
4.8/5  (37)
(37)
What is the concentration in moles per liter of pure PbSO4(s) given that it has a density of 6.29 g/cm3?
(Multiple Choice)
4.8/5  (44)
(44)
 -What happens with respect to time to the products of this reaction in the kinetic region of the graph above?
-What happens with respect to time to the products of this reaction in the kinetic region of the graph above?
(Multiple Choice)
4.8/5  (43)
(43)
Calculate the concentrations of Cl2 and ClF3 at equilibrium if a reaction that initially contained 1.000 M concentrations of both Cl2 and F2 is found to have an F2 concentration of 0.700 M at equilibrium. 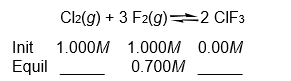
(Multiple Choice)
4.8/5  (41)
(41)
In which of the following will the least PbBr2(s) dissolve?
(Multiple Choice)
4.8/5  (33)
(33)
If the equilibrium constant for the following reaction is Kc = 1 x 102
2 C2H6(g) + 7 O2(g) 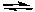 4 CO2(g) + 6 H2O(g)
And all the concentrations were initially 0.10 M, we can predict that the reaction
4 CO2(g) + 6 H2O(g)
And all the concentrations were initially 0.10 M, we can predict that the reaction
(Multiple Choice)
4.8/5  (35)
(35)
Under which set of conditions must the following reaction always proceed to the right in order to reach equilibrium?
H2(g) + I2(g) 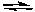 2 HI(g)
2 HI(g)
(Multiple Choice)
4.8/5  (36)
(36)
Which is the correct equilibrium constant expression for the reaction:
2 SO2(g) + O2(g) 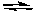 2 SO3(g)
2 SO3(g)
(Multiple Choice)
4.8/5  (27)
(27)
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 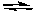 2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-Starting with 0.10 M H2O(g), what would be the best strategy for solving for the equilibrium concentrations of H2O, H2 and O2?
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-Starting with 0.10 M H2O(g), what would be the best strategy for solving for the equilibrium concentrations of H2O, H2 and O2?
(Multiple Choice)
4.7/5  (31)
(31)
Calculate the COCl2, CO, and Cl2 concentrations when the following gas-phase reaction reaches equilibrium at 300°C.
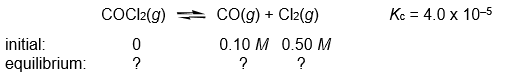
(Essay)
4.8/5  (32)
(32)
Which of the following factors will cause this chemical reaction to shift its equilibrium to the right?
Xe(g) + 2 F2(g) 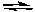 XeF4(g) H = -218 kJ/molrxn
XeF4(g) H = -218 kJ/molrxn
(Multiple Choice)
4.9/5  (43)
(43)
For the following reaction:
2 NOCl(g) 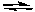 2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO2) = 0.989 M at some moment in time, which of the following would be true?
2 NO2(g) + Cl2(g) Kc = 5.6 x 10-6 (at 400 K)
If (NOCl) = 0.0222 M, (Cl2) = 0.0222 M, and (NO2) = 0.989 M at some moment in time, which of the following would be true?
(Multiple Choice)
4.9/5  (36)
(36)
Based on the information given in the previous problem, the equilibrium constant for the following reaction would be:
2IBr(g) 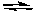 I2(g) + Br2(g)
I2(g) + Br2(g)
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following graphs best represents the relationship between the concentration of reactants and products with respect to time for the following chemical reaction?
2 H2(g) + O2(g) 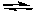 2 H2O(g)
2 H2O(g)
(Multiple Choice)
4.9/5  (40)
(40)
Assume that the equilibrium constant for the following reaction is known.
2 NO2(g) 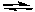 2 NO(g) + O2(g) K1 = 7.4 x 10-16 (at 25°C)
What is the correct value of the equilibrium constant for the opposite reaction?
2 NO(g) + O2(g)
2 NO(g) + O2(g) K1 = 7.4 x 10-16 (at 25°C)
What is the correct value of the equilibrium constant for the opposite reaction?
2 NO(g) + O2(g) 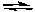 2 NO2(g) K2 = ?
2 NO2(g) K2 = ?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)