Deck 12: Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 12: Thermodynamics
1
Two processes are shown on the p-V graph in Figure 12-1; one is an adiabat and the other is an isotherm. Is the process represented by the upper curve an adiabat or an isotherm?
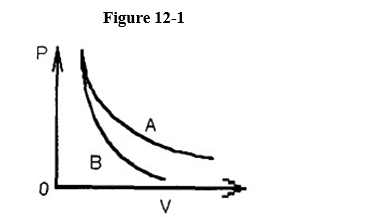

isotherm
2
Why don't we use "efficiency" for rating refrigerators, like we rate engines, instead of the Coefficient of Performance?
In both cases we desire a ratio of "what we want" divided by "what it costs"; for a refrig. this is "heat absorbed"/"work input" (COP) but for an engine this is "work out"/"heat in" (= eff).
3
Is it possible for heat to travel from a cold object to a hotter object?
Yes (your refrigerator does this all the time), but only with the expenditure of work.
4
We say that energy cannot be created nor destroyed, yet a heat pump usually delivers more energy (heat) into the house than it receives from the power source (electricity, gas, etc). Why is this not a violation of the law of conservation of energy?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
Both the refrigerator and the heat pump take in heat and expel it at a higher temperature. Why don't they have the same Coefficient of Performances?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
As a system loses the ability to do work, its entropy decreases.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
Entropy is a measure of order. The more order there is, the higher the system's entropy.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
Entropy of the universe is increasing.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
A Carnot cycle requires an ideal gas for its "working substance."

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
Change in internal energy is
A) Qout/Win.
B) 1 - Tcold/Thot.
C) 1 - Qcold/Qhot.
D) Q/m.
E) Q - W.
A) Qout/Win.
B) 1 - Tcold/Thot.
C) 1 - Qcold/Qhot.
D) Q/m.
E) Q - W.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
The First Law of Thermodynamics is equivalent to
A) the first law of motion.
B) Newton's third law of motion.
C) the law of conservation of momentum.
D) the law of conservation of energy.
E) the Equipartition Principle.
A) the first law of motion.
B) Newton's third law of motion.
C) the law of conservation of momentum.
D) the law of conservation of energy.
E) the Equipartition Principle.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle.
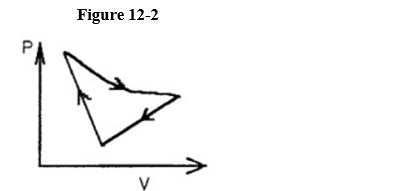
If the process was carried out in a clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-2, then the change of internal energy over the full cycle
A) is positive.
B) is negative.
C) is zero.
D) cannot be determined from the information given.

If the process was carried out in a clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-2, then the change of internal energy over the full cycle
A) is positive.
B) is negative.
C) is zero.
D) cannot be determined from the information given.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
The change of the internal energy of an ideal gas depends upon
A) changing temperature.
B) changing volume.
C) changing pressure and volume together.
D) changing pressure.
A) changing temperature.
B) changing volume.
C) changing pressure and volume together.
D) changing pressure.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
A substance is taken through the illustrated cycle in Figure 12-3 from
(p1,v1) to (3 p1,2 v1) to (p1,2 v1) back to (p1, v1).
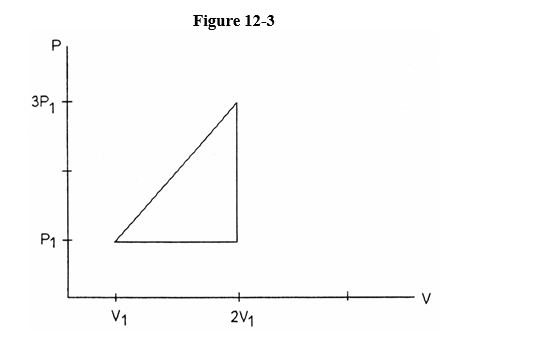
How much work was done?
A) 3 p1v1
B) 5 p1v1
C) p1v1
D) 4 p1v1
E) 2 p1v1
(p1,v1) to (3 p1,2 v1) to (p1,2 v1) back to (p1, v1).

How much work was done?
A) 3 p1v1
B) 5 p1v1
C) p1v1
D) 4 p1v1
E) 2 p1v1

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
A cyclic process is carried out on an ideal gas such that it returns to its initial
State at the end of a cycle.
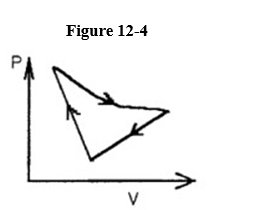
If the process was carried out on a clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-4, then that area represents
A) the heat that flows from the ideal gas.
B) the work done on the ideal gas.
C) the heat added to the ideal gas.
D) the work done by the ideal gas.
State at the end of a cycle.

If the process was carried out on a clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-4, then that area represents
A) the heat that flows from the ideal gas.
B) the work done on the ideal gas.
C) the heat added to the ideal gas.
D) the work done by the ideal gas.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle.
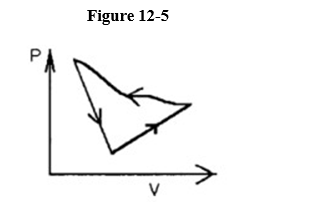
If the process was carried out in a counter-clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-5, then that area represents
A) the work done on the ideal gas.
B) the heat added to the ideal gas.
C) the work done by the ideal gas.
D) the heat that flows from the ideal gas.

If the process was carried out in a counter-clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-5, then that area represents
A) the work done on the ideal gas.
B) the heat added to the ideal gas.
C) the work done by the ideal gas.
D) the heat that flows from the ideal gas.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
A gas is confined to a rigid container that cannot expand as heat energy is added to it. This process is
A) isothermal.
B) isobaric.
C) adiabatic.
D) isometric.
E) isentropic.
A) isothermal.
B) isobaric.
C) adiabatic.
D) isometric.
E) isentropic.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
A gas is allowed to expand at constant pressure as heat is added to it. This process is
A) isothermal.
B) isentropic.
C) adiabatic.
D) isobaric.
E) isometric.
A) isothermal.
B) isentropic.
C) adiabatic.
D) isobaric.
E) isometric.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
The process shown on the p-V graph in Figure 12-6 is an
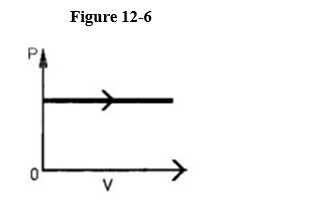
A) isobaric expansion.
B) isothermal expansion.
C) adiabatic expansion.
D) isometric expansion.

A) isobaric expansion.
B) isothermal expansion.
C) adiabatic expansion.
D) isometric expansion.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
The process shown on the p-V graph in Figure 12-7 is an
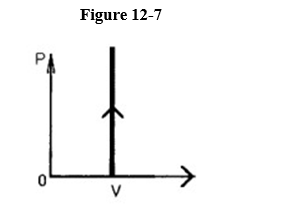
A) isotherm.
B) adiabatic.
C) isometric.
D) isobaric.

A) isotherm.
B) adiabatic.
C) isometric.
D) isobaric.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
The process shown on the T-V graph in Figure 12-8 is an
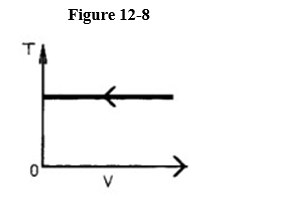
A) isobaric compression.
B) isometric compression.
C) isothermal compression.
D) adiabatic compression.

A) isobaric compression.
B) isometric compression.
C) isothermal compression.
D) adiabatic compression.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
Isobaric work is
A) p T.
B) Q - W.
C) Q + W.
D) p V.
E) V p.
A) p T.
B) Q - W.
C) Q + W.
D) p V.
E) V p.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
When the first law of thermodynamics, Q = u + W, is applied to an ideal gas that is taken through an adiabatic process,
A) u = 0.
B) it is not true that u = 0 or W = 0 or Q = 0.
C) Q = 0.
D) W = 0.
A) u = 0.
B) it is not true that u = 0 or W = 0 or Q = 0.
C) Q = 0.
D) W = 0.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
The work done by the gas is zero during an
A) adiabatic expansion.
B) isothermal expansion.
C) isobaric expansion.
D) isometric process.
A) adiabatic expansion.
B) isothermal expansion.
C) isobaric expansion.
D) isometric process.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
A gas is expanded to twice its original volume with no change in its temperature. This process is
A) isothermal.
B) adiabatic.
C) isobaric.
D) isometric.
E) isentropic.
A) isothermal.
B) adiabatic.
C) isobaric.
D) isometric.
E) isentropic.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
A gas is quickly compressed in an isolated environment. During the event, the gas exchanged no heat with its surroundings. This process is
A) isothermal.
B) isobaric.
C) adiabatic.
D) isometric.
A) isothermal.
B) isobaric.
C) adiabatic.
D) isometric.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
The work done by the gas is negative for an
A) adiabatic expansion.
B) isothermal compression.
C) isometric process.
D) isothermal expansion.
A) adiabatic expansion.
B) isothermal compression.
C) isometric process.
D) isothermal expansion.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
Consider two cylinders of gas identical in all respects except that one contains O2 and the other He. Both hold the same volume of gas at STP and are closed by a movable piston at one end. Both gases are now compressed adiabatically to one-third their original volume. Which gas will show the greater temperature increase?
A) the O2
B) impossible to tell from the information given
C) neither; both will show the same increase
D) the He
A) the O2
B) impossible to tell from the information given
C) neither; both will show the same increase
D) the He

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
An ideal gas is compressed to one-half its original volume during an isothermal process. The final pressure of the gas
A) does not change.
B) increases to less than twice its original value.
C) increases to more than twice its original value.
D) increases to twice its original value.
A) does not change.
B) increases to less than twice its original value.
C) increases to more than twice its original value.
D) increases to twice its original value.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
A monatomic ideal gas is cooled to one-half its original temperature during an isometric process. The final pressure of the gas
A) increases to twice its original value.
B) does not change.
C) increases to more than twice its original value.
D) decreases to half its original value.
A) increases to twice its original value.
B) does not change.
C) increases to more than twice its original value.
D) decreases to half its original value.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
When the first law of thermodynamics, Q = u + W, is applied to an ideal gas that is taken through an isothermal process,
A) P = 0.
B) W = 0.
C) Q = 0.
D) u = 0.
A) P = 0.
B) W = 0.
C) Q = 0.
D) u = 0.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
When the first law of thermodynamics, Q = u + W, is applied to an ideal gas that is taken through an isobaric process,
A) Q = 0.
B) it is not true that u = 0 or W = 0 or Q = 0.
C) u = 0.
D) W = 0.
A) Q = 0.
B) it is not true that u = 0 or W = 0 or Q = 0.
C) u = 0.
D) W = 0.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
Consider two cylinders of gas identical in all respects except that one contains O2 and the other He. Both hold the same volume of gas at STP and are closed by a movable piston at one end. Both gases are now compressed adiabatically to one-third their original volume. Which gas will show the greater pressure increase?
A) impossible to tell from the information given
B) neither; both will show the same increase
C) the O2
D) the He
A) impossible to tell from the information given
B) neither; both will show the same increase
C) the O2
D) the He

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
When temperature is plotted against entropy, the area under the process path is equal to
A) the heat transferred.
B) the change in internal energy.
C) the work done.
D) the heat added less the work done.
A) the heat transferred.
B) the change in internal energy.
C) the work done.
D) the heat added less the work done.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
As a system loses its ability to do useful work
A) entropy decreases.
B) energy increases.
C) entropy remains constant.
D) entropy increases.
E) energy decreases.
A) entropy decreases.
B) energy increases.
C) entropy remains constant.
D) entropy increases.
E) energy decreases.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
The statement that "heat energy cannot be completely transformed into work" is a statement of which thermodynamic law?
A) second
B) fourth
C) first
D) third
E) zeroth
A) second
B) fourth
C) first
D) third
E) zeroth

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
Is it possible to transfer heat from a hot reservoir to a cold reservoir?
A) yes, but work will have to be done
B) yes; this will happen naturally
C) no!
D) theoretically yes, but it hasn't been accomplished yet
A) yes, but work will have to be done
B) yes; this will happen naturally
C) no!
D) theoretically yes, but it hasn't been accomplished yet

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
The change in entropy is Q/T for
A) irreversible processes.
B) reversible processes.
C) all processes.
D) exothermic processes.
E) all real engines.
A) irreversible processes.
B) reversible processes.
C) all processes.
D) exothermic processes.
E) all real engines.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
When water freezes, the entropy of the water
A) could either increase or decrease; it depends on other factors.
B) does not change.
C) decreases.
D) increases.
A) could either increase or decrease; it depends on other factors.
B) does not change.
C) decreases.
D) increases.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
Thermal efficiency (non-Carnot) is
A) Q/T.
B) 1 - Tcold/Thot.
C) 1 - Qcold/Qhot.
D) Q/m.
E) Q - W.
A) Q/T.
B) 1 - Tcold/Thot.
C) 1 - Qcold/Qhot.
D) Q/m.
E) Q - W.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
Coefficient of performance is
A) Qout/Win.
B) Q - W.
C) 1 - Tcold/Thot.
D) Q/m.
E) 1 - Qcold/Qhot.
A) Qout/Win.
B) Q - W.
C) 1 - Tcold/Thot.
D) Q/m.
E) 1 - Qcold/Qhot.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
Only Carnot efficiency is
A) 1 - Qhot/Qcold.
B) 1 - Qcold/Qhot.
C) 1 - Tcold/Thot.
D) 1 - Thot/Tcold.
E) Qout/Win.
A) 1 - Qhot/Qcold.
B) 1 - Qcold/Qhot.
C) 1 - Tcold/Thot.
D) 1 - Thot/Tcold.
E) Qout/Win.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
The most efficient engine possible is the
A) Kelvin cycle.
B) Otto cycle.
C) Joule cycle.
D) Wright cycle.
E) Carnot cycle.
A) Kelvin cycle.
B) Otto cycle.
C) Joule cycle.
D) Wright cycle.
E) Carnot cycle.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
An example of a reversible process is the
A) Swinn cycle.
B) Wenkle cycle.
C) Carnot cycle.
D) Otto cycle.
E) Joule cycle.
A) Swinn cycle.
B) Wenkle cycle.
C) Carnot cycle.
D) Otto cycle.
E) Joule cycle.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
Being the ideal engine, the maximum COP of a Carnot cycle operated in reverse as a refrigerator is
A) (1 - Tc/Th)-1.
B) (Th/Tc)(1-Tc/Th)-1.
C) 100.
D) (Tc/Th) . (1-Tc/Th)-1.
A) (1 - Tc/Th)-1.
B) (Th/Tc)(1-Tc/Th)-1.
C) 100.
D) (Tc/Th) . (1-Tc/Th)-1.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
Referring to Figure 12-9, a substance carried from point A to B absorbs 50. J and finds its internal energy has increased by 20. J. Going from B to C the internal energy decreases by 5. Joules.
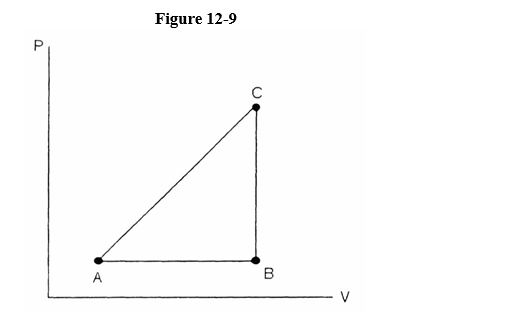
(a) How much work was done from A to B?
(b) How much heat was absorbed from B to C?
(c) How much work was done going from B to C?

(a) How much work was done from A to B?
(b) How much heat was absorbed from B to C?
(c) How much work was done going from B to C?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
250. J of work is done in compressing a gas adiabatically. What is the change in internal energy of the gas?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
A piece of metal at 80°C is placed in 1.2 L of water at 72°C. The system is thermally isolated and reaches a final temperature of 75°C. Estimate the approximate change in entropy for this process.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
A container of ideal gas at STP undergoes an isothermal expansion and its entropy changes by 3.66 J/°K. How much work does it do?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
A coal-fired plant generates 600 MW of electric power. The plant uses 4.8 × 106 kg of coal each day. The heat of combustion of coal is 3.3 × 107 J/kg. The steam that drives the turbines is at a temperature of 300°C, and the exhaust water is at 37°C. How much thermal energy is wasted each day?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
One of the most efficient engines built so far has the following characteristics: combustion chamber temperature = 1900°C; exhaust temperature = 430°C. 7 × 109 cal of fuel produces 1.4 × 1010J of work in one hour. What is the power output, in hp, of this engine?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
What is the maximum theoretical efficiency possible for an engine operating between 100° C and 400° C?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
A heat engine operating between 40° C and 380° C has an efficiency 60% of that of a Carnot engine operating between the same temperatures. If the engine absorbs heat at a rate of 60 kW, at what rate does it exhaust heat?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
One of the most efficient engines built so far has the following characteristics:
combustion chamber temperature = 1900°C; exhaust temperature = 430°C.
7 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour.
(a) What is the actual efficiency of this engine?
(b) What is the Carnot efficiency of this engine?
combustion chamber temperature = 1900°C; exhaust temperature = 430°C.
7 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour.
(a) What is the actual efficiency of this engine?
(b) What is the Carnot efficiency of this engine?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
A Carnot refrigerator has a COP of 2.0.
(a) What is its efficiency operated as an engine?
(b) Operated as an engine, heat is taken in at 500.°C; what is the exhaust Celsius temperature?
(a) What is its efficiency operated as an engine?
(b) Operated as an engine, heat is taken in at 500.°C; what is the exhaust Celsius temperature?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
A coal-fired plant generates 600 MW of electric power. The plant uses 4.8 × 106 kg of coal each day. The heat of combustion of coal is 3.3 × 107 J/kg. The steam that drives the turbines is at a temperature of 300°C, and the exhaust water is at 37°C.
(a) What is the overall efficiency of the plant for generating electric power?
(b) What is the Carnot efficiency?
(a) What is the overall efficiency of the plant for generating electric power?
(b) What is the Carnot efficiency?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
A certain amount of a monatomic gas is maintained at constant volume as it is cooled by 50 K. This feat is accomplished by removing 400 J of energy from the gas. How much work is done by the gas?
A) zero
B) -400 J
C) -200 J
D) 400 J
E) 200 J
A) zero
B) -400 J
C) -200 J
D) 400 J
E) 200 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
A gas is taken through the cycle illustrated here in Figure 12-10.
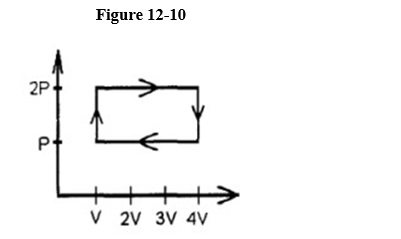
During one cycle, how much work is done by an engine operating on this cycle?
A) 2pV
B) 4pV
C) pV
D) 5pV
E) 3pV

During one cycle, how much work is done by an engine operating on this cycle?
A) 2pV
B) 4pV
C) pV
D) 5pV
E) 3pV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
An ideal gas is compressed isothermally from 30 L to 20 L. During this process, 6 J of energy is expended by the external mechanism that compressed the gas. What is the change of internal energy for this gas?
A) zero
B) -6 J
C) +12 J
D) -12 J
E) +6 J
A) zero
B) -6 J
C) +12 J
D) -12 J
E) +6 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
An ideal gas is expanded isothermally from 20 L to 30 L. During this process, 6 J of energy is expended by the external mechanism that expanded the gas. Which of the following statements is correct?
A) 6 J of energy flow from the gas into the surroundings.
B) 6 J of energy flow from surroundings into the gas.
C) No energy flows into or from the gas since this process is isothermal.
D) None of the above statements is correct.
A) 6 J of energy flow from the gas into the surroundings.
B) 6 J of energy flow from surroundings into the gas.
C) No energy flows into or from the gas since this process is isothermal.
D) None of the above statements is correct.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
What is the change of entropy associated with 3.0 kg of water freezing to ice at 0.°C?
A) -0.88 kcal/K
B) 1.0 kcal/K
C) -1.1 kcal/K
D) +0.88 kcal/K
A) -0.88 kcal/K
B) 1.0 kcal/K
C) -1.1 kcal/K
D) +0.88 kcal/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
In each cycle an engine gets 50. J from the combustion of fuel and it expels 45. J of heat to the environment at 13.°C. What is the minimum possible temperature at which the 50. J could be taken in?
A) 33°C
B) 45°C
C) 23°C
D) 27°C
E) 20°C
A) 33°C
B) 45°C
C) 23°C
D) 27°C
E) 20°C

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
The Department of Energy develops a new reversible engine which has a COP of 4 when operated as a refrigerator and a COP of 5 when operated as a heat pump. What is its efficiency when operated as an engine doing work?
A) 45.%
B) 20.%
C) 80.%
D) 10.%
E) 25.%
A) 45.%
B) 20.%
C) 80.%
D) 10.%
E) 25.%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
The Otto cycle has how many strokes per cycle during which the volume decreases?
A) 4
B) 8
C) 3
D) 2
E) 1
A) 4
B) 8
C) 3
D) 2
E) 1

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
A reversible engine takes in 40. Joules of heat, does 10. J of work, and expels 30. J of wasted heat during each cycle. If it is operated in reverse as a refrigerator, then its COP would be
A) 75%.
B) 25%.
C) 4.
D) 3.
E) 4%.
A) 75%.
B) 25%.
C) 4.
D) 3.
E) 4%.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
Consider a Carnot refrigerator which is operated between -3°C and 23.°C. What is its COP?
A) 10.
B) 3.1
C) 9.0
D) 11.
E) 10%
A) 10.
B) 3.1
C) 9.0
D) 11.
E) 10%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
A Carnot engine is operated as a refrigerator, taking in 18. J of heat every second and expelling 20. J into a 23.°C room. What is the temperature inside the refrigerator?
A) -6.6°C
B) 3.0°C
C) -15°C
D) 15°C
E) 300. K
A) -6.6°C
B) 3.0°C
C) -15°C
D) 15°C
E) 300. K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
A Carnot cycle is operated in reverse as a refrigerator taking in 36. J every minute and "dumping" 40. J per minute. at a room temperature of 23.°C. The net entropy increase per minute is
A) 0.13 J/°K
B) zero
C) 36.J/°K
D) 0.15 J/°K
E) 0.15 kJ/°K
A) 0.13 J/°K
B) zero
C) 36.J/°K
D) 0.15 J/°K
E) 0.15 kJ/°K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
If the theoretical efficiency of a Carnot engine is to be 100%, the heat sink must be
A) at absolute zero.
B) infinitely hot.
C) at 100°C.
D) at 0°C.
E) a perfect radiator.
A) at absolute zero.
B) infinitely hot.
C) at 100°C.
D) at 0°C.
E) a perfect radiator.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
What is the theoretical efficiency of a Carnot engine that operates between 600 K and 300 K?
A) 100%
B) 75%
C) 25%
D) 50%
E) 0%
A) 100%
B) 75%
C) 25%
D) 50%
E) 0%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck


