Exam 12: Thermodynamics
Exam 1: Measurement and Problem Solving98 Questions
Exam 2: Kinematics: Description of Motion92 Questions
Exam 3: Motion in Two Dimensions104 Questions
Exam 4: Force and Motion64 Questions
Exam 5: Work and Energy62 Questions
Exam 6: Linear Momentum and Collisions71 Questions
Exam 7: Circular Motion and Gravitation93 Questions
Exam 8: Rotational Motion and Equilibrium68 Questions
Exam 9: Solids and Fluids75 Questions
Exam 10: Temperature and Kinetic Theory68 Questions
Exam 11: Heat61 Questions
Exam 12: Thermodynamics70 Questions
Exam 13: Vibrations and Waves79 Questions
Exam 14: Sound61 Questions
Exam 15: Electric Charge, Forces, and Fields46 Questions
Exam 16: Electric Potential, Energy, and Capacitance47 Questions
Exam 17: Electric Current and Resistance59 Questions
Exam 18: Basic Electric Circuits68 Questions
Exam 19: Magnetism58 Questions
Exam 20: Electromagnetic Induction and Waves43 Questions
Exam 21: AC Circuits50 Questions
Exam 22: Reflection and Refraction of Light53 Questions
Exam 23: Mirrors and Lenses61 Questions
Exam 24: Physical Optics: The Wave Nature of Light57 Questions
Exam 25: Vision and Optical Instruments53 Questions
Exam 26: Relativity60 Questions
Exam 27: Quantum Physics58 Questions
Exam 28: Quantum Mechanics and Atomic Physics54 Questions
Exam 29: The Nucleus63 Questions
Exam 30: Nuclear Reactions and Elementary Particles54 Questions
Select questions type
We say that energy cannot be created nor destroyed, yet a heat pump usually delivers more energy (heat) into the house than it receives from the power source (electricity, gas, etc). Why is this not a violation of the law of conservation of energy?
Free
(Essay)
4.8/5  (33)
(33)
Correct Answer:
The extra energy was extracted from some environmental source like the outside air or from the ground.
A coal-fired plant generates 600 MW of electric power. The plant uses 4.8 × 106 kg of coal each day. The heat of combustion of coal is 3.3 × 107 J/kg. The steam that drives the turbines is at a temperature of 300°C, and the exhaust water is at 37°C. How much thermal energy is wasted each day?
Free
(Short Answer)
4.9/5  (37)
(37)
Correct Answer:
1.1 × 1014 J
An ideal gas is compressed to one-half its original volume during an isothermal process. The final pressure of the gas
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
D
A reversible engine takes in 40. Joules of heat, does 10. J of work, and expels 30. J of wasted heat during each cycle. If it is operated in reverse as a refrigerator, then its COP would be
(Multiple Choice)
4.8/5  (32)
(32)
A heat engine operating between 40° C and 380° C has an efficiency 60% of that of a Carnot engine operating between the same temperatures. If the engine absorbs heat at a rate of 60 kW, at what rate does it exhaust heat?
(Short Answer)
4.8/5  (31)
(31)
A cyclic process is carried out on an ideal gas such that it returns to its initial
State at the end of a cycle.
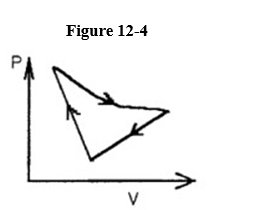 If the process was carried out on a clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-4, then that area represents
If the process was carried out on a clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-4, then that area represents
(Multiple Choice)
4.8/5  (24)
(24)
A certain amount of a monatomic gas is maintained at constant volume as it is cooled by 50 K. This feat is accomplished by removing 400 J of energy from the gas. How much work is done by the gas?
(Multiple Choice)
4.8/5  (32)
(32)
Is it possible for heat to travel from a cold object to a hotter object?
(Essay)
4.8/5  (31)
(31)
An ideal gas is compressed isothermally from 30 L to 20 L. During this process, 6 J of energy is expended by the external mechanism that compressed the gas. What is the change of internal energy for this gas?
(Multiple Choice)
5.0/5  (29)
(29)
A coal-fired plant generates 600 MW of electric power. The plant uses 4.8 × 106 kg of coal each day. The heat of combustion of coal is 3.3 × 107 J/kg. The steam that drives the turbines is at a temperature of 300°C, and the exhaust water is at 37°C.
(a) What is the overall efficiency of the plant for generating electric power?
(b) What is the Carnot efficiency?
(Short Answer)
4.9/5  (30)
(30)
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle.
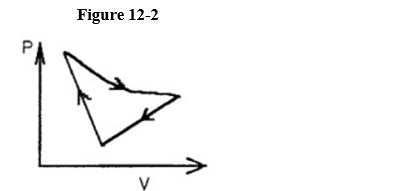 If the process was carried out in a clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-2, then the change of internal energy over the full cycle
If the process was carried out in a clockwise sense around the enclosed area, as shown on the p-V diagram in Figure 12-2, then the change of internal energy over the full cycle
(Multiple Choice)
4.8/5  (37)
(37)
The Otto cycle has how many strokes per cycle during which the volume decreases?
(Multiple Choice)
4.7/5  (36)
(36)
The Department of Energy develops a new reversible engine which has a COP of 4 when operated as a refrigerator and a COP of 5 when operated as a heat pump. What is its efficiency when operated as an engine doing work?
(Multiple Choice)
4.9/5  (43)
(43)
Showing 1 - 20 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)