Deck 11: States of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/65
Play
Full screen (f)
Deck 11: States of Matter
1
Which of the following statements concerning the physical states of the elements at room temperature (25º C) and pressure is incorrect?
A) Only two elements, bromine and mercury, are liquids.
B) The vast majority of the elements are solids.
C) The gaseous state is more common than the liquid state.
D) Over half of all the elements are liquids.
A) Only two elements, bromine and mercury, are liquids.
B) The vast majority of the elements are solids.
C) The gaseous state is more common than the liquid state.
D) Over half of all the elements are liquids.
Over half of all the elements are liquids.
2
Which best describes the size and shape of a sample of liquid?
A) definite volume and definite shape
B) volume and shape both determined by the container
C) definite volume, but shape is determined by the container
D) volume determined by container, but definite shape
A) definite volume and definite shape
B) volume and shape both determined by the container
C) definite volume, but shape is determined by the container
D) volume determined by container, but definite shape
definite volume, but shape is determined by the container
3
Gases are highly compressible because the particles ________.
A) strongly repel each other
B) have no potential energy
C) are very far apart
D) vibrate too much to be compressed
A) strongly repel each other
B) have no potential energy
C) are very far apart
D) vibrate too much to be compressed
are very far apart
4
Which of the following is not a correct characterization of the liquid state?
A) The particles move relatively long distances before colliding with adjacent particles.
B) The particles are randomly packed in relatively close proximity to each other.
C) The particles "slide" freely over each other.
D) The particles do not have sufficient energy to separate from each other.
A) The particles move relatively long distances before colliding with adjacent particles.
B) The particles are randomly packed in relatively close proximity to each other.
C) The particles "slide" freely over each other.
D) The particles do not have sufficient energy to separate from each other.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
5
In the solid state disruptive forces are ________.
A) roughly of the same magnitude as cohesive forces
B) very weak compared to cohesive forces
C) dominant over cohesive forces
D) a consequence of excessive potential energy.
A) roughly of the same magnitude as cohesive forces
B) very weak compared to cohesive forces
C) dominant over cohesive forces
D) a consequence of excessive potential energy.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
6
According to kinetic molecular theory, the molecules of a gas ________.
A) are constantly moving in a regular pattern
B) are constantly moving in a random manner
C) cannot move because of lack of space
D) move only at high temperatures (above 1000
A) are constantly moving in a regular pattern
B) are constantly moving in a random manner
C) cannot move because of lack of space
D) move only at high temperatures (above 1000

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
7
Which is not characteristic of liquids?
A) Collisions between particles occur often.
B) Particles are essentially independent of one another.
C) Under normal pressures, the particles are relatively close together.
D) The particles are not very compressible when pressure is applied.
A) Collisions between particles occur often.
B) Particles are essentially independent of one another.
C) Under normal pressures, the particles are relatively close together.
D) The particles are not very compressible when pressure is applied.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
8
When liquids and gases are compared, liquids have ________ compressability compared to gases and a ________ density.
A) greater, smaller
B) smaller, greater
C) smaller, smaller
D) greater, greater
A) greater, smaller
B) smaller, greater
C) smaller, smaller
D) greater, greater

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
9
Snow forms in the clouds when water vapor freezes without ever passing through the liquid phase. This process is known as ________.
A) sublimation
B) condensation
C) freezing
D) deposition
A) sublimation
B) condensation
C) freezing
D) deposition

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following terms does not involve the solid state?
A) evaporation
B) melting
C) freezing
D) sublimation
A) evaporation
B) melting
C) freezing
D) sublimation

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following quantities is needed in calculating the amount of heat energy released as water turns to ice at 0 °C?
A) the mass and heat of solidification
B) heat of fusion for water and the mass
C) heat of vaporization for water and the mass
D) heat of condensation for water and the mass
A) the mass and heat of solidification
B) heat of fusion for water and the mass
C) heat of vaporization for water and the mass
D) heat of condensation for water and the mass

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
12
How many Joules of heat must be removed to lower the temperature of a 36.5 g Al bar from 84.1 °C to 56.8 °C? The specific heat of Al is 0.908 J/g °C.
A) 581 J
B) 1090 J
C) 905 J
D) 240 J
A) 581 J
B) 1090 J
C) 905 J
D) 240 J

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
13
The specific heat of substance A is one fourth that of substance B. The temperature of both a 24.0 g sample of substance A and a 12.0 g sample of substance B was raised 20 °C. The heat absorbed by substance A was ________ the heat absorbed by substance B.
A) equal to
B) one-half
C) twice
D) four times
A) equal to
B) one-half
C) twice
D) four times

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
14
How much heat energy in Joules is required to heat 16.0 g of copper from 23.0 °C to 66.1 °C? Specific heat of Cu = 0.382 J/(g °C)
A) 263 J
B) 812 J
C) 450 J
D) 109 J
A) 263 J
B) 812 J
C) 450 J
D) 109 J

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
15
A sample of aluminum absorbed 9.86 J of heat and the temperature increased from 23.2 °C to 30.5 °C. What is mass of the aluminum? The specific heat of aluminum is 0.90 J/g °C.
A) 72 g
B) 1.5 g
C) 65 g
D) 8.1 g
A) 72 g
B) 1.5 g
C) 65 g
D) 8.1 g

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
16
What is the final temperature after 336 J of heat energy is removed from 25.0 g of water at 19.6 °C? Specific heat of water = 4.18 J/(g °C)
A) 32.1 °C
B) 27.6 °C
C) 16.4 °C
D) 24.7 °C
A) 32.1 °C
B) 27.6 °C
C) 16.4 °C
D) 24.7 °C

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
17
What is the heat capacity of 84.0 g of water? Specific heat of water = 4.18 J/(g °C)
A) 351 J/°C
B) 96.8 J/°C
C) 506 J/°C
D) 492 J/°C
A) 351 J/°C
B) 96.8 J/°C
C) 506 J/°C
D) 492 J/°C

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
18
How many grams of Ag can be heated from 23 °C to 36 °C when 22 g of Au cools from 95.5 °C to 26.4 °C?
Specific heat of Ag = 0.240 J/(g °C) Specific heat of Au = 0.130 J/(g °C)
A) 47 g
B) 28 g
C) 63 g
D) 104 g
Specific heat of Ag = 0.240 J/(g °C) Specific heat of Au = 0.130 J/(g °C)
A) 47 g
B) 28 g
C) 63 g
D) 104 g

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the mass of gold (0.130 J/g °C) that requires 468 J to heat the metal from 21.6 °C to 33.2 °C?
A) 56.9 g
B) 622 g
C) 538 g
D) 310 g
A) 56.9 g
B) 622 g
C) 538 g
D) 310 g

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
20
A T-bone steak provides 6.90 x 102 food Calories (Cal). A food Calorie is equivalent to 4180 J of heat energy. The heat energy provided by the steak would be sufficient to heat 59.1 kg of water how many Celsius degrees. The specific heat of water is 4.18 J/g °C.
A) 6.58 °C
B) 11.7 °C
C) 9.20 °C
D) 23.6 C
A) 6.58 °C
B) 11.7 °C
C) 9.20 °C
D) 23.6 C

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
21
The combustion of one mole of a gas produces 212 calories of energy. Express the energy released in this reaction in kilojoules.
A) 239 kJ
B) 886 kJ
C) 0.886 kJ
D) 0.239 kJ
A) 239 kJ
B) 886 kJ
C) 0.886 kJ
D) 0.239 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements concerning temperature change as a substance is heated is incorrect?
A) As a solid is heated, its temperature rises until its melting point is reached.
B) During the time a solid melts to a liquid the temperature remains constant.
C) As a liquid is heated, its temperature rises until its boiling point is reached.
D) During the time a liquid is changing to the gaseous state the temperature gradually increases while all the liquid is changed.
A) As a solid is heated, its temperature rises until its melting point is reached.
B) During the time a solid melts to a liquid the temperature remains constant.
C) As a liquid is heated, its temperature rises until its boiling point is reached.
D) During the time a liquid is changing to the gaseous state the temperature gradually increases while all the liquid is changed.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following would be correct units for a heat of condensation value?
A) J/°C
B) Cal/°C
C) g/°C
D) Cal/g
A) J/°C
B) Cal/°C
C) g/°C
D) Cal/g

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following would have the same numerical magnitude?
A) heats of fusion and deposition
B) heats of sublimation and condensation
C) heats of sublimation and deposition
D) heats of solidification and condensation
A) heats of fusion and deposition
B) heats of sublimation and condensation
C) heats of sublimation and deposition
D) heats of solidification and condensation

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following pairs of quantities is needed in calculating the amount of heat energy needed to change liquid water at 75 °C to steam at 110 °C?
A) specific heat of water, specific heat of steam, and heat of vaporization for water
B) specific heat of ice and heat of fusion for water
C) heat of fusion for water and heat of condensation for water
D) specific heat of ice and specific heat of water
A) specific heat of water, specific heat of steam, and heat of vaporization for water
B) specific heat of ice and heat of fusion for water
C) heat of fusion for water and heat of condensation for water
D) specific heat of ice and specific heat of water

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
26
How much energy is required to change 12.9 g of solid Cu to molten Cu at 1083 °C (melting point)?
Heat of fusion for Cu = 205 J/g
A) 1390
B) 3150
C) 2640
D) 1990
Heat of fusion for Cu = 205 J/g
A) 1390
B) 3150
C) 2640
D) 1990

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
27
How much heat must be absorbed to evaporate 14 g of NH3 from liquid to gas state at ?33 °C (condensation point)? Heat of condensation for NH3 = 1380 J/g
A) 19,000 J
B) 91,000 J
C) 46,000 J
D) 62,000 J
A) 19,000 J
B) 91,000 J
C) 46,000 J
D) 62,000 J

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
28
What is the heat of vaporization in J/g of an unknown liquid if 6823 J of heat are required to vaporize 58.0 g of the unknown at its boiling point?
A) 516 J/g
B) 118 J/g
C) 981 J/g
D) 28,100 J/g
A) 516 J/g
B) 118 J/g
C) 981 J/g
D) 28,100 J/g

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the heat energy that must be removed from 17.6 grams of ammonia gas to condense it to liquid ammonia at its boiling point, ?33 °C. The heat of vaporization for ammonia is 1380 J/g.
A) 24,300 J
B) 78.4 J
C) 0.0128 J
D) 9,680 J
A) 24,300 J
B) 78.4 J
C) 0.0128 J
D) 9,680 J

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
30
How much heat energy is needed to convert 10.0 g of ice at ?10 °C to liquid water at 10 °C?
Specific heat of ice = 2.09 J/(g°C) Heat of fusion of ice = 334 J/g Specific heat of water = 4.18 J/g°C
A) 5270 J
B) 1,070 J
C) 6,210 J
D) 3960 J
Specific heat of ice = 2.09 J/(g°C) Heat of fusion of ice = 334 J/g Specific heat of water = 4.18 J/g°C
A) 5270 J
B) 1,070 J
C) 6,210 J
D) 3960 J

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
31
) In the calculation set-up below to determine how much heat is needed to convert 50 g of ice at ?20 °C to steam at 300 °C, which constant does C represent?
(A)(50 g)(20 °C) + (heat of fusion)(50 g) + (B)(50 g)(100 °C) + (heat of vap.)(50 g) + (C)(50 g)(200 °C) = total heat
A) specific heat of ice
B) specific heat of water
C) specific heat of steam
D) heat capacity of water
(A)(50 g)(20 °C) + (heat of fusion)(50 g) + (B)(50 g)(100 °C) + (heat of vap.)(50 g) + (C)(50 g)(200 °C) = total heat
A) specific heat of ice
B) specific heat of water
C) specific heat of steam
D) heat capacity of water

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
32
The calculation below is set up to determine how much heat is needed to convert 50 g of ice at ?20 °C to steam at 300 °C. Which constant does A represent? (A)(50 g)(20 °C) + (heat of fusion)(50 g) + (4.18 J/g°C)(50 g)(100 °C) + (B)(50 g) + (C)(50 g )(200 °C) = total heat
A) heat of condensation
B) specific heat of ice
C) specific heat of water
D) heat of vaporization of water
A) heat of condensation
B) specific heat of ice
C) specific heat of water
D) heat of vaporization of water

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements concerning evaporation is correct?
A) Molecules with energies considerably above average escape from the liquid.
B) Decreasing the surface area of the liquid increases the rate of evaporation.
C) Evaporation causes the liquid temperature to remain constant.
D) Increasing the surface area of the liquid decreases the rate of evaporation.
A) Molecules with energies considerably above average escape from the liquid.
B) Decreasing the surface area of the liquid increases the rate of evaporation.
C) Evaporation causes the liquid temperature to remain constant.
D) Increasing the surface area of the liquid decreases the rate of evaporation.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
34
A nonvolatile liquid would ________.
A) have weak attractive forces between molecules
B) have a high vapor pressure at room temperature
C) be a very "explosive" substance
D) have strong attractive forces between molecules
A) have weak attractive forces between molecules
B) have a high vapor pressure at room temperature
C) be a very "explosive" substance
D) have strong attractive forces between molecules

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
35
The vapor pressure of SnCl4 reaches 400 mm Hg at 92 °C, the vapor pressure of SnI4 reaches 400 mm Hg at 315 °C, the vapor pressure of PBr3 reaches 400 mm Hg at 150 °C, and the vapor pressure of PCl3 reaches 400 mm Hg at 57 °C. At 175 °C which substance would have the lowest vapor pressure?
A) PBr3
B) SnI4
C) SnCl4
D) PCl3
A) PBr3
B) SnI4
C) SnCl4
D) PCl3

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
36
What effect will decreasing the temperature of a liquid by 15 °C at constant pressure have on the magnitude of its vapor pressure?
A) increase
B) decrease
C) no change
D) Insufficient information given to determine.
A) increase
B) decrease
C) no change
D) Insufficient information given to determine.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
37
Which statement about the boiling point of water is incorrect?
A) The boiling point is greater than 100 °C in a pressure cooker.
B) The boiling point is less than 100 °C for locations at low elevations.
C) At sea level and at a pressure of 760 mm Hg, the boiling point is 100 °C.
D) In a pressure cooker, shorter cooking times are required due to the change in boiling point.
A) The boiling point is greater than 100 °C in a pressure cooker.
B) The boiling point is less than 100 °C for locations at low elevations.
C) At sea level and at a pressure of 760 mm Hg, the boiling point is 100 °C.
D) In a pressure cooker, shorter cooking times are required due to the change in boiling point.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
38
The normal boiling point of a substance is determined by its molecular mass and its intermolecular forces. Considering these two factors, predict the order of increasing boiling points for the following substances: CH4, CS2, KBr, NH3.
A) CS2 < CH4 < KBr < NH3
B) CH4 < CS2 < NH3 < KBr
C) NH3 < KBr < CS2 < CH4
D) CH4 < KBr < NH3 < CS2
A) CS2 < CH4 < KBr < NH3
B) CH4 < CS2 < NH3 < KBr
C) NH3 < KBr < CS2 < CH4
D) CH4 < KBr < NH3 < CS2

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following compounds would have the lowest boiling point?
A) CH4
B) NO2
C) HF
D) LiCl
A) CH4
B) NO2
C) HF
D) LiCl

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
40
On the basis of your understanding of bonding in liquids and solids, arrange the following substances from the highest to lowest melting points: NaCl Na Cl2 SiO2
A) Cl2, Na, NaCl, SiO2
B) Na, NaCl, Cl2, SiO2
C) SiO2, NaCl, Na, Cl2
D) NaCl, SiO2, Na, Cl2
A) Cl2, Na, NaCl, SiO2
B) Na, NaCl, Cl2, SiO2
C) SiO2, NaCl, Na, Cl2
D) NaCl, SiO2, Na, Cl2

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
41
The strength of London dispersion forces depends on what two factors?
A) molecular mass and polarizability
B) vapor pressure and size
C) molecular mass and volatility
D) volatility and shape
A) molecular mass and polarizability
B) vapor pressure and size
C) molecular mass and volatility
D) volatility and shape

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
42
In which of the following liquids would dipole-dipole interactions be the predominant intermolecular force?
A) NH3
B) HCl
C) PF3
D) both B and C
A) NH3
B) HCl
C) PF3
D) both B and C

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following kinds of intermolecular forces is found in all molecules?
A) hydrogen bonding
B) dipole-dipole interactions
C) London forces
D) Chicago forces
A) hydrogen bonding
B) dipole-dipole interactions
C) London forces
D) Chicago forces

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following substances cannot form hydrogen bonds?
A) HF
B) CH3NH2
C) HCl
D) NH3
A) HF
B) CH3NH2
C) HCl
D) NH3

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
45
The boiling points of the halogens increase going from fluorine to iodine. What type of intermolecular force(s) is (are) responsible for this trend?
A) London dispersion forces
B) hydrogen bonding
C) ion-dipole attraction
D) dipole-dipole interactions
A) London dispersion forces
B) hydrogen bonding
C) ion-dipole attraction
D) dipole-dipole interactions

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following substances would be expected to have the highest boiling point?
A) a nonpolar liquid
B) a polar liquid with hydrogen bonding
C) a polar liquid with weak dipole-dipole interactions
D) a nonvolatile liquid
A) a nonpolar liquid
B) a polar liquid with hydrogen bonding
C) a polar liquid with weak dipole-dipole interactions
D) a nonvolatile liquid

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following would have the highest boiling point?
A) CO2
B) H2O
C) CH4
D) Kr
A) CO2
B) H2O
C) CH4
D) Kr

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
48
The anomalously high boiling point of water is due to ________.
A) London forces
B) its low heat of vaporization
C) hydrogen bonding
D) ice being less dense than liquid water
A) London forces
B) its low heat of vaporization
C) hydrogen bonding
D) ice being less dense than liquid water

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
49
In a liquid sample of NCl3, what is the dominant intermolecular force present?
A) London forces
B) hydrogen bonding
C) instantaneous bonding
D) dipole-dipole interactions
A) London forces
B) hydrogen bonding
C) instantaneous bonding
D) dipole-dipole interactions

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
50
If liquid methyl amine and hydrofluoric acid were mixed, between which two atoms in these molecules would a hydrogen bond form?
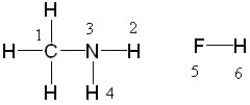
A) between 4 and 5, between 2 and 5, or between 3 and 6
B) between 2 and 5 only
C) between 3 and 6 only
D) between 4 and 5 or 2 and 5

A) between 4 and 5, between 2 and 5, or between 3 and 6
B) between 2 and 5 only
C) between 3 and 6 only
D) between 4 and 5 or 2 and 5

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
51
If liquid ammonia and water were mixed, between which two atoms in these molecules would a hydrogen bond form?
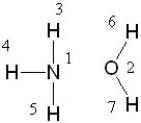
A) between 1 and 3
B) between 2 and 6
C) between 3 and 2, 4 and 2, 5 and 2, 1 and 6, and 1 and 7
D) Hydrogen bond formation is not possible between these two molecules.

A) between 1 and 3
B) between 2 and 6
C) between 3 and 2, 4 and 2, 5 and 2, 1 and 6, and 1 and 7
D) Hydrogen bond formation is not possible between these two molecules.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following molecules are capable of hydrogen bonding with water?
H2S NH3 H2 HBr CH
A) NH3, HBr, and H2
B) NH3 and HF
C) H2S and CH4
D) H2S, HBr, and H2
H2S NH3 H2 HBr CH
A) NH3, HBr, and H2
B) NH3 and HF
C) H2S and CH4
D) H2S, HBr, and H2

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following types of crystalline solids would be expected to have the lowest melting point?
A) polar molecular
B) nonpolar molecular
C) macromolecular
D) ionic
A) polar molecular
B) nonpolar molecular
C) macromolecular
D) ionic

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
54
The crystal lattice sites in a solid ________.
A) are always occupied by molecules
B) may be occupied by atoms
C) cannot be occupied by ions
D) cannot be occupied by nonpolar molecules
A) are always occupied by molecules
B) may be occupied by atoms
C) cannot be occupied by ions
D) cannot be occupied by nonpolar molecules

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
55
In which of the following types of crystalline solids would the forces between particles be covalent bonds?
A) covalent network
B) ionic
C) metallic
D) polar molecular
A) covalent network
B) ionic
C) metallic
D) polar molecular

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
56
Which one of the following is classified as a covalent solid?
A) K2SO4
B) Cr
C) CO2
D) C
A) K2SO4
B) Cr
C) CO2
D) C

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
57
What would be the temperature change, in C, if 555 J of heat energy is added to 35.0 g of a metal with a specific heat of 0.418 J/g °C?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
58
A chocolate, caramel pecan piece of candy contains 115 food Calories (Cal). A food Calorie is equivalent to 1000 calories of heat energy. If the heat energy contained in the piece of candy were transferred to 53.5 kg of water at 25.0 °C, what would be the final temperature of the water? The specific heat of water is 4.18 J/g °C.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
59
The specific heat of lead is 0.13 J/g °C. How many joules of heat would be required to raise the temperature of 25.0 g of lead from 21 °C to 39 °C?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
60
The ClF molecule is polar. Use + and − signs to indicate the direction of polarity in the ClF bond. Using a dashed line, illustrate an ion-dipole interaction between a ClF molecule and a lithium ion.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
61
Use a dashed line to illustrate hydrogen bonding between a methanol molecule and a hydrogen fluoride molecule.
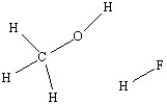


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
62
Predict the predominate intermolecular force (London force, dipole-dipole, or hydrogen bonding) that would be associated with each of the following compounds.
-BeF2 ______________________________
-BeF2 ______________________________

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
63
Predict the predominate intermolecular force (London force, dipole-dipole, or hydrogen bonding) that would be associated with each of the following compounds.
-OF2 ______________________________
-OF2 ______________________________

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
64
Predict the predominate intermolecular force (London force, dipole-dipole, or hydrogen bonding) that would be associated with each of the following compounds.
-HCl ______________________________
-HCl ______________________________

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
65
Predict the predominate intermolecular force (London force, dipole-dipole, or hydrogen bonding) that would be associated with each of the following compounds.
-HCOOH ______________________________
-HCOOH ______________________________

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck


