Exam 11: States of Matter
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
Which of the following substances cannot form hydrogen bonds?
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
C
The boiling points of the halogens increase going from fluorine to iodine. What type of intermolecular force(s) is (are) responsible for this trend?
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
A
The specific heat of lead is 0.13 J/g °C. How many joules of heat would be required to raise the temperature of 25.0 g of lead from 21 °C to 39 °C?
Free
(Essay)
4.9/5  (39)
(39)
Correct Answer:
q = SH × m × (Tf - Ti) = 0.13 J/g C × 25.0 g × (39 - 21) = 58000 J = 58 kJ
In which of the following liquids would dipole-dipole interactions be the predominant intermolecular force?
(Multiple Choice)
4.7/5  (37)
(37)
Predict the predominate intermolecular force (London force, dipole-dipole, or hydrogen bonding) that would be associated with each of the following compounds.
-OF2 ______________________________
(Short Answer)
4.9/5  (35)
(35)
When liquids and gases are compared, liquids have ________ compressability compared to gases and a ________ density.
(Multiple Choice)
4.7/5  (37)
(37)
Snow forms in the clouds when water vapor freezes without ever passing through the liquid phase. This process is known as ________.
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following terms does not involve the solid state?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following compounds would have the lowest boiling point?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following would have the highest boiling point?
(Multiple Choice)
4.8/5  (37)
(37)
How many grams of Ag can be heated from 23 °C to 36 °C when 22 g of Au cools from 95.5 °C to 26.4 °C?
Specific heat of Ag = 0.240 J/(g °C) Specific heat of Au = 0.130 J/(g °C)
(Multiple Choice)
4.9/5  (33)
(33)
The ClF molecule is polar. Use + and − signs to indicate the direction of polarity in the ClF bond. Using a dashed line, illustrate an ion-dipole interaction between a ClF molecule and a lithium ion.
(Essay)
4.8/5  (39)
(39)
If liquid ammonia and water were mixed, between which two atoms in these molecules would a hydrogen bond form?
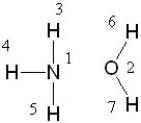
(Multiple Choice)
4.9/5  (51)
(51)
Which of the following statements concerning evaporation is correct?
(Multiple Choice)
4.9/5  (42)
(42)
In which of the following types of crystalline solids would the forces between particles be covalent bonds?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following kinds of intermolecular forces is found in all molecules?
(Multiple Choice)
4.8/5  (41)
(41)
What is the final temperature after 336 J of heat energy is removed from 25.0 g of water at 19.6 °C? Specific heat of water = 4.18 J/(g °C)
(Multiple Choice)
5.0/5  (38)
(38)
Predict the predominate intermolecular force (London force, dipole-dipole, or hydrogen bonding) that would be associated with each of the following compounds.
-HCOOH ______________________________
(Short Answer)
4.9/5  (44)
(44)
Calculate the heat energy that must be removed from 17.6 grams of ammonia gas to condense it to liquid ammonia at its boiling point, ?33 °C. The heat of vaporization for ammonia is 1380 J/g.
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 65
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)