Deck 4: Basic Concepts About Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 4: Basic Concepts About Matter
1
Which of the following is a property of both solids and liquids?
A) definite shape
B) indefinite shape
C) definite volume
D) indefinite volume
A) definite shape
B) indefinite shape
C) definite volume
D) indefinite volume
definite volume
2
Gallium has a melting point of 30 °C and a boiling point of 2403 °C. At which temperature below will gallium be a gas?
A) -25 °C
B) 25 °C
C) 250 °C
D) 2500 °C
A) -25 °C
B) 25 °C
C) 250 °C
D) 2500 °C
2500 °C
3
Which statement best describes a sample of gas?
A) A gas has a definite volume and a definite shape.
B) The volume and the shape of a gas are determined by its container.
C) A gas has a definite volume, but its shape is determined by its container.
D) The volume of a gas is determined by its container, but it has a definite shape.
A) A gas has a definite volume and a definite shape.
B) The volume and the shape of a gas are determined by its container.
C) A gas has a definite volume, but its shape is determined by its container.
D) The volume of a gas is determined by its container, but it has a definite shape.
The volume and the shape of a gas are determined by its container.
4
The state of matter of a substance is determined by its ________.
A) electrical conductivity
B) density
C) temperature
D) solubility
A) electrical conductivity
B) density
C) temperature
D) solubility

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
Molten iron metal has a(n) ________ volume and a(n) ________ shape.
A) definite; definite
B) indefinite; indefinite
C) definite; indefinite
D) indefinite; definite
A) definite; definite
B) indefinite; indefinite
C) definite; indefinite
D) indefinite; definite

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
Nitrogen gas has a(n) ________ volume and a(n) ________ shape.
A) definite; definite
B) indefinite; definite
C) definite; indefinite
D) indefinite; indefinite
A) definite; definite
B) indefinite; definite
C) definite; indefinite
D) indefinite; indefinite

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements does not describe a physical property?
A) Sparks are created when sodium metal is added to water.
B) A strip of magnesium metal weighs 7.49 grams.
C) Snow forms in the clouds via deposition.
D) Mercury is a liquid at room temperature.
A) Sparks are created when sodium metal is added to water.
B) A strip of magnesium metal weighs 7.49 grams.
C) Snow forms in the clouds via deposition.
D) Mercury is a liquid at room temperature.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
A solid substance is subjected to a number of tests and observations. Which of the following test results would not be classified as a physical property of the substance?
A) It reacts with base to form water.
B) Its density is 1.84 g/mL.
C) It tastes sour.
D) It is a white-colored solid.
A) It reacts with base to form water.
B) Its density is 1.84 g/mL.
C) It tastes sour.
D) It is a white-colored solid.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
In which of the following pairs are both chemical properties?
A) has metallic luster, soluble in ammonia
B) flammable, reacts with acid
C) green in color, reacts violently with water
D) has a high density, is very brittle
A) has metallic luster, soluble in ammonia
B) flammable, reacts with acid
C) green in color, reacts violently with water
D) has a high density, is very brittle

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a chemical change?
A) rusting of iron
B) dissolving salt in water
C) evaporation of gasoline
D) cutting a copper wire into two pieces
A) rusting of iron
B) dissolving salt in water
C) evaporation of gasoline
D) cutting a copper wire into two pieces

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is a chemical property?
A) condensation
B) mass
C) solubility
D) burning
A) condensation
B) mass
C) solubility
D) burning

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
When a substance undergoes a chemical change it is always true that ________.
A) it condenses
B) it changes state
C) new substances are formed
D) heat is absorbed
A) it condenses
B) it changes state
C) new substances are formed
D) heat is absorbed

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is not a chemical change?
A) frying an egg
B) rusting of iron
C) slicing a ham
D) burning a candle
A) frying an egg
B) rusting of iron
C) slicing a ham
D) burning a candle

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following represents a physical change in matter?
A) A substance melts when heated.
B) A substance gives off a gas when heated.
C) A substance burns when heated.
D) A substance remains unchanged as it is heated.
A) A substance melts when heated.
B) A substance gives off a gas when heated.
C) A substance burns when heated.
D) A substance remains unchanged as it is heated.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is a chemical change?
A) distilling ocean water
B) making tea from a tea bag
C) rusting of iron
D) melting of snow
A) distilling ocean water
B) making tea from a tea bag
C) rusting of iron
D) melting of snow

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a physical change?
A) burning coal
B) synthesis of methane from carbon and hydrogen
C) smoking a cigarette
D) filtering muddy water
A) burning coal
B) synthesis of methane from carbon and hydrogen
C) smoking a cigarette
D) filtering muddy water

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
The condensation of steam is an example of a ________.
A) change of state
B) extensive property
C) sublimation
D) chemical change
A) change of state
B) extensive property
C) sublimation
D) chemical change

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
Which response includes all of the following that involve chemical changes and no physical changes?
I. burning charcoal
II. melting of gold
III. fermentation of grapes
IV. burning of natural gas
V. melting of ice
A) II and V
B) I and IV
C) I, II, and III
D) I, III, and IV
I. burning charcoal
II. melting of gold
III. fermentation of grapes
IV. burning of natural gas
V. melting of ice
A) II and V
B) I and IV
C) I, II, and III
D) I, III, and IV

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
As two liquid solutions are added together, a yellow solid forms. This change is most likely:
A) a physical change
B) a chemical change
C) neither
D) both
A) a physical change
B) a chemical change
C) neither
D) both

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
Which response contains all of the following that are physical changes and no chemical changes?
I. Candle wax melts.
II. Skin turns yellow when it comes in contact with nitric acid.
III. Milk curdles.
IV. Ice floats on water.
V. Windows fog when the temperature drops rapidly.
A) II and IV
B) I, III, and V
C) II and III
D) I, IV, and V
I. Candle wax melts.
II. Skin turns yellow when it comes in contact with nitric acid.
III. Milk curdles.
IV. Ice floats on water.
V. Windows fog when the temperature drops rapidly.
A) II and IV
B) I, III, and V
C) II and III
D) I, IV, and V

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
Which answer includes all of the following that are chemical changes, and no physical changes?
I. The cutting of an iron bar into smaller pieces.
II. The solidification of mercury by cooling.
III. The dropping of iron into hydrochloric acid, producing hydrogen gas.
IV. The mixing of water and drinking alcohol.
V. The fermentation of grape juice.
A) II, III, IV, and V
B) III and V
C) I, III, and IV
D) I and IV
I. The cutting of an iron bar into smaller pieces.
II. The solidification of mercury by cooling.
III. The dropping of iron into hydrochloric acid, producing hydrogen gas.
IV. The mixing of water and drinking alcohol.
V. The fermentation of grape juice.
A) II, III, IV, and V
B) III and V
C) I, III, and IV
D) I and IV

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
Which of these is an intensive property?
A) temperature
B) mass
C) length
D) volume
A) temperature
B) mass
C) length
D) volume

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
Which of these is an extensive property?
A) density
B) color
C) mass
D) melting point
A) density
B) color
C) mass
D) melting point

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is not a pure substance?
A) blood
B) calcium
C) sodium bicarbonate
D) distilled water
A) blood
B) calcium
C) sodium bicarbonate
D) distilled water

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
A liter sample of oxygen has been purified. Which of the following characteristics will not apply to the oxygen sample?
A) Only oxygen is present in the sample.
B) The sample can be separated into two parts by physical means.
C) All molecules in the sample will be composed of two atoms, (O2).
D) At 25 °C and 1 atm pressure, all portions of the sample will have the same properties.
A) Only oxygen is present in the sample.
B) The sample can be separated into two parts by physical means.
C) All molecules in the sample will be composed of two atoms, (O2).
D) At 25 °C and 1 atm pressure, all portions of the sample will have the same properties.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following can be classified as a mixture and not as a pure substance?
I. oxygen
II. antifreeze
III. distilled water
IV. human blood
V. brass alloy
A) II and V
B) II and III
C) I only
D) II, IV, and V
I. oxygen
II. antifreeze
III. distilled water
IV. human blood
V. brass alloy
A) II and V
B) II and III
C) I only
D) II, IV, and V

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
The description "three substances present, two phases present" is correct for ________.
A) heterogeneous mixtures
B) homogeneous mixtures
C) elements
D) compounds
A) heterogeneous mixtures
B) homogeneous mixtures
C) elements
D) compounds

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
Which is an example of a homogeneous mixture?
A) chocolate chip cookie
B) a rock collection
C) glucose solution
D) oil & vinegar salad dressing
A) chocolate chip cookie
B) a rock collection
C) glucose solution
D) oil & vinegar salad dressing

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
A pure substance has been isolated in the laboratory. Based on the two characteristics below, how can the substance be classified?
I. The species cannot be separated by physical means.
II. The species can be separated by chemical means.
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture
I. The species cannot be separated by physical means.
II. The species can be separated by chemical means.
A) an element
B) a compound
C) a homogeneous mixture
D) a heterogeneous mixture

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
Which statement is false?
A) Different mixtures of the same two substances must have the same composition.
B) A mixture of sea sand and table salt is an example of a heterogeneous mixture.
C) An example of a homogeneous mixture is one prepared by mixing drinking alcohol and water.
D) A compound is a substance that can be decomposed into two or more simpler substances by chemical means.
A) Different mixtures of the same two substances must have the same composition.
B) A mixture of sea sand and table salt is an example of a heterogeneous mixture.
C) An example of a homogeneous mixture is one prepared by mixing drinking alcohol and water.
D) A compound is a substance that can be decomposed into two or more simpler substances by chemical means.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following substances is a heterogeneous mixture?
A) sodium
B) baking soda
C) dextrose (a sugar) dissolved in water
D) salt and pepper in a glass
A) sodium
B) baking soda
C) dextrose (a sugar) dissolved in water
D) salt and pepper in a glass

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a heterogeneous mixture?
A) ice and water
B) tap water
C) pure water
D) gasoline
A) ice and water
B) tap water
C) pure water
D) gasoline

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following type of matter/classification of matter pairs is incorrectly matched?
A) Type of matter: pure gold
Classification of matter: element
B) Type of matter: concrete
Classification of matter: homogeneous mixture
C) Type of matter: fruitcake
Classification of matter: heterogeneous mixture
D) Type of matter: saline solution
Classification of matter: homogeneous mixture
A) Type of matter: pure gold
Classification of matter: element
B) Type of matter: concrete
Classification of matter: homogeneous mixture
C) Type of matter: fruitcake
Classification of matter: heterogeneous mixture
D) Type of matter: saline solution
Classification of matter: homogeneous mixture

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
Choose the incorrect response concerning the substances listed below.
I. iron
II. neon
III. iodized table salt
IV. cola
V. steam
A) III can be decomposed by chemical means.
B) III and IV represent mixtures.
C) I and II represent elements.
D) I, II, III, IV and V represent pure substances.
I. iron
II. neon
III. iodized table salt
IV. cola
V. steam
A) III can be decomposed by chemical means.
B) III and IV represent mixtures.
C) I and II represent elements.
D) I, II, III, IV and V represent pure substances.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
If a tablespoon of table salt is mixed with a tablespoon of water, the resulting solution can be classified as a ________.
A) homogeneous mixture
B) pure substance
C) homogeneous pure substance
D) heterogeneous mixture
A) homogeneous mixture
B) pure substance
C) homogeneous pure substance
D) heterogeneous mixture

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is a compound?
A) copper
B) magnesium
C) copper (II) oxide
D) iron
A) copper
B) magnesium
C) copper (II) oxide
D) iron

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
A pure substance A is found to change upon heating into two new pure substances, B and C. C can be decomposed by chemical means, B cannot be decomposed by chemical means. From this we may conclude that ________.
A) A is an element, B and C are compounds
B) A is a compound, B and C are elements
C) A, B and C are all elements
D) A and C are compounds, B is an element
A) A is an element, B and C are compounds
B) A is a compound, B and C are elements
C) A, B and C are all elements
D) A and C are compounds, B is an element

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements about compounds and mixtures is correct?
A) Since two or more simpler substances can be obtained from a compound, all compounds are mixtures.
B) Compounds cannot be broken down into simpler substances by chemical means.
C) Mixtures retain the properties of their individual components.
D) Compounds always have properties that are identical to those of the elements used to produce them.
A) Since two or more simpler substances can be obtained from a compound, all compounds are mixtures.
B) Compounds cannot be broken down into simpler substances by chemical means.
C) Mixtures retain the properties of their individual components.
D) Compounds always have properties that are identical to those of the elements used to produce them.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
A pure substance A is found to change upon heating into two new, pure substances, B and C.
A B + C
Neither B nor C can be decomposed by chemical means. From this we may conclude that ________.
A) A is an element, B and C are compounds
B) B is a compound, A and C are elements
C) B and C are elements, A is a compound
D) A, B, and C are all compounds
A B + C
Neither B nor C can be decomposed by chemical means. From this we may conclude that ________.
A) A is an element, B and C are compounds
B) B is a compound, A and C are elements
C) B and C are elements, A is a compound
D) A, B, and C are all compounds

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
In which of the following time periods was the greatest number of elements discovered?
A) ancient - 1700
B) 1701 - 1800
C) 1801 - 1900
D) 1901 - present
A) ancient - 1700
B) 1701 - 1800
C) 1801 - 1900
D) 1901 - present

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following statements concerning the known elements is true?
A) 88 of the elements are naturally occurring.
B) No new elements have been identified within the last twenty years.
C) 104 elements are known at present.
D) Scientists "suspect" that there are more naturally occurring elements to be discovered.
A) 88 of the elements are naturally occurring.
B) No new elements have been identified within the last twenty years.
C) 104 elements are known at present.
D) Scientists "suspect" that there are more naturally occurring elements to be discovered.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
The most abundant elements in the universe are ________.
A) oxygen and iron
B) hydrogen and helium
C) helium and carbon
D) hydrogen and oxygen
A) oxygen and iron
B) hydrogen and helium
C) helium and carbon
D) hydrogen and oxygen

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
The most abundant elements in the Earth's crust are ________.
A) oxygen and silicon
B) oxygen and carbon
C) hydrogen and helium
D) hydrogen and oxygen
A) oxygen and silicon
B) oxygen and carbon
C) hydrogen and helium
D) hydrogen and oxygen

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
In which of the following sequences of elements do each of the elements have a one-letter symbol?
A) sulfur, vanadium, cobalt
B) chlorine, iron, lithium
C) nickel, yttrium, potassium
D) hydrogen, fluorine, phosphorous
A) sulfur, vanadium, cobalt
B) chlorine, iron, lithium
C) nickel, yttrium, potassium
D) hydrogen, fluorine, phosphorous

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
In which of the following sequences of elements do each of the elements have a two-letter symbol?
A) sodium, lithium, iodine
B) potassium, fluorine, carbon
C) tin, hydrogen, iodine
D) silicon, chlorine, chromium
A) sodium, lithium, iodine
B) potassium, fluorine, carbon
C) tin, hydrogen, iodine
D) silicon, chlorine, chromium

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
In which of the following sequences of elements do each of the elements have a symbol which starts with a letter not the first letter of the element's English name?
A) silver, gold, mercury
B) copper, helium, neon
C) cobalt, chromium, sodium
D) argon, iron, lead
A) silver, gold, mercury
B) copper, helium, neon
C) cobalt, chromium, sodium
D) argon, iron, lead

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
Which statement is true about elements?
A) Elements have similar symbols and names.
B) Element names are derived either from geographical names or from the plants.
C) The first letter of any chemical symbol is capitalized.
D) The elements whose symbols are derived from non-English names are the recently found elements.
A) Elements have similar symbols and names.
B) Element names are derived either from geographical names or from the plants.
C) The first letter of any chemical symbol is capitalized.
D) The elements whose symbols are derived from non-English names are the recently found elements.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
In which pair does the symbol not match the name of the element?
A) nitrogen - N
B) iron - Fe
C) strontium - Sr
D) arsenic - Ar
A) nitrogen - N
B) iron - Fe
C) strontium - Sr
D) arsenic - Ar

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
In which pair does the symbol match the element name?
A) strontium - St
B) chlorine - Cl
C) gallium - Gl
D) tungsten - Th
A) strontium - St
B) chlorine - Cl
C) gallium - Gl
D) tungsten - Th

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
How many of the following pairs of elements and symbols are entirely correct?
Silver, Si Manganese, Mg Copper, Co Potassium, P
A) none
B) 1
C) 2
D) 3
Silver, Si Manganese, Mg Copper, Co Potassium, P
A) none
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
How many total atoms are contained in the following formula: KAl(SO4)2 ?
A) 4
B) 12
C) 10
D) 7
A) 4
B) 12
C) 10
D) 7

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
Indicate whether each of the following statements represents a chemical change, a physical change, or no change occurred.
-Sodium metal is cut with a knife. ____________________
-Sodium metal is cut with a knife. ____________________

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
Indicate whether each of the following statements represents a chemical change, a physical change, or no change occurred.
-Hydrochloric acid was added to baking sodaresulting in the evolution of carbon dioxide gas. ____________________
-Hydrochloric acid was added to baking sodaresulting in the evolution of carbon dioxide gas. ____________________

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
Indicate whether each of the following statements represents a chemical change, a physical change, or no change occurred.
-A red piece of copper wire is added to a beakercontaining hydrochloric acid (a clear, colorless solution).After an hour a red wire is observed in a clear,colorless solution. ____________________
-A red piece of copper wire is added to a beakercontaining hydrochloric acid (a clear, colorless solution).After an hour a red wire is observed in a clear,colorless solution. ____________________

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
Indicate whether each of the following statements represents a chemical change, a physical change, or no change occurred.
-A chunk of cheddar cheese is grated. ____________________
-A chunk of cheddar cheese is grated. ____________________

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
Indicate whether each of the following statements represents a chemical change, a physical change, or no change occurred.
-Candle wax is melted. ____________________
-Candle wax is melted. ____________________

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
Indicate whether each of the following statements represents a chemical change, a physical change, or no change occurred.
-Candle wax is burned. ____________________
-Candle wax is burned. ____________________

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
What is the difference between an extensive and an intensive property? Give one example of each.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 2 substances present, 3 phases present
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element
- 2 substances present, 3 phases present
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 2 substances present, 2 phases present
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element
- 2 substances present, 2 phases present
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 2 substances present, 1 phase present
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element
- 2 substances present, 1 phase present
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 1 substance present, 2 phases present, substance cannot be decomposed by chemical means
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element
- 1 substance present, 2 phases present, substance cannot be decomposed by chemical means
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 1 substance present, 1 phase present, substance can be decomposed by chemical means
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element
- 1 substance present, 1 phase present, substance can be decomposed by chemical means
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 1 substance present, 1 phase present, substance cannot be decomposed by chemical means
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element
- 1 substance present, 1 phase present, substance cannot be decomposed by chemical means
A) heterogeneous mixture
B) homogeneous mixture
C) compound
D) element

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
Indicate whether the following descriptions of matter represents an
-One substance present, one phase present, substancecannot be decomposed by chemical means. ____________________
A) element
B) mixture
C) compound
-One substance present, one phase present, substancecannot be decomposed by chemical means. ____________________
A) element
B) mixture
C) compound

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
Indicate whether the following descriptions of matter represents an
-Two substances present, two phases present. ____________________
A) element
B) mixture
C) compound
-Two substances present, two phases present. ____________________
A) element
B) mixture
C) compound

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
Indicate whether the following descriptions of matter represents an
-One substance present, two elements present. ____________________
A) element
B) mixture
C) compound
-One substance present, two elements present. ____________________
A) element
B) mixture
C) compound

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
68
Indicate whether the following descriptions of matter represents an
-Two elements present, composition is variable. ____________________
A) element
B) mixture
C) compound
-Two elements present, composition is variable. ____________________
A) element
B) mixture
C) compound

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
69
The schematic below illustrates the breakdown of a compound via chemical means. Identify the products (as elements or compounds) formed at the end of chemical means 1 and the products formed at the end of chemical means 2.
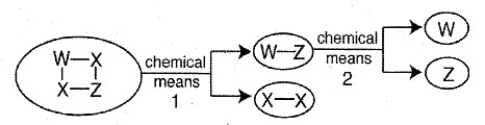


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
For each set of elements choose the appropriate characterization of the set's elemental symbols from the response list. Responses may be used more than once or need not be used at all.
- neon, lithium, scandium
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name
- neon, lithium, scandium
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
For each set of elements choose the appropriate characterization of the set's elemental symbols from the response list. Responses may be used more than once or need not be used at all.
- iron, tungsten, potassium
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name
- iron, tungsten, potassium
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
For each set of elements choose the appropriate characterization of the set's elemental symbols from the response list. Responses may be used more than once or need not be used at all.
- oxygen, fluorine, tungsten
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name
- oxygen, fluorine, tungsten
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
For each set of elements choose the appropriate characterization of the set's elemental symbols from the response list. Responses may be used more than once or need not be used at all.
- cesium, chlorine, copper
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name
- cesium, chlorine, copper
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
For each set of elements choose the appropriate characterization of the set's elemental symbols from the response list. Responses may be used more than once or need not be used at all.
- gold, silver, arsenic
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name
- gold, silver, arsenic
A) all symbols have one letter
B) all symbols have two letters
C) all symbols start with the same letter
D) all symbols start with a letter not the first letter of the element's English name

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
Fill in the missing chemical symbol or element name for each of the following pairs.
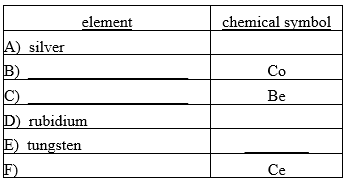


Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck


