Exam 4: Basic Concepts About Matter
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
Which is an example of a homogeneous mixture?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
C
Which of the following is a property of both solids and liquids?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
C
As two liquid solutions are added together, a yellow solid forms. This change is most likely:
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
D
Which of the following statements about compounds and mixtures is correct?
(Multiple Choice)
4.8/5  (33)
(33)
Indicate whether the following descriptions of matter represents an
-One substance present, one phase present, substancecannot be decomposed by chemical means. ____________________
(Multiple Choice)
4.9/5  (34)
(34)
Indicate whether each of the following statements represents a chemical change, a physical change, or no change occurred.
-A red piece of copper wire is added to a beakercontaining hydrochloric acid (a clear, colorless solution).After an hour a red wire is observed in a clear,colorless solution. ____________________
(Short Answer)
4.7/5  (41)
(41)
In which pair does the symbol not match the name of the element?
(Multiple Choice)
4.7/5  (41)
(41)
Gallium has a melting point of 30 °C and a boiling point of 2403 °C. At which temperature below will gallium be a gas?
(Multiple Choice)
4.7/5  (36)
(36)
In which of the following sequences of elements do each of the elements have a symbol which starts with a letter not the first letter of the element's English name?
(Multiple Choice)
4.9/5  (45)
(45)
Indicate whether each of the following statements represents a chemical change, a physical change, or no change occurred.
-Candle wax is burned. ____________________
(Short Answer)
4.9/5  (37)
(37)
A solid substance is subjected to a number of tests and observations. Which of the following test results would not be classified as a physical property of the substance?
(Multiple Choice)
4.9/5  (33)
(33)
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 1 substance present, 1 phase present, substance can be decomposed by chemical means
(Multiple Choice)
4.7/5  (40)
(40)
Choose the incorrect response concerning the substances listed below.
I. iron
II. neon
III. iodized table salt
IV. cola
V. steam
(Multiple Choice)
4.8/5  (41)
(41)
For each characterization of matter, choose the appropriate classification from the response list. Responses may be used more than once or need not be used at all.
- 2 substances present, 3 phases present
(Multiple Choice)
4.8/5  (34)
(34)
A pure substance has been isolated in the laboratory. Based on the two characteristics below, how can the substance be classified?
I. The species cannot be separated by physical means.
II. The species can be separated by chemical means.
(Multiple Choice)
4.8/5  (33)
(33)
Fill in the missing chemical symbol or element name for each of the following pairs.
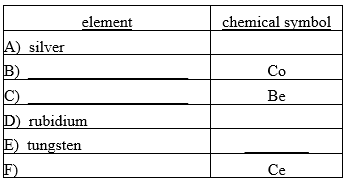
(Essay)
4.8/5  (28)
(28)
Indicate whether each of the following statements represents a chemical change, a physical change, or no change occurred.
-A chunk of cheddar cheese is grated. ____________________
(Short Answer)
4.9/5  (48)
(48)
Showing 1 - 20 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)