Deck 3: Unit Systems and Dimensional Analysis
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 3: Unit Systems and Dimensional Analysis
1
In which of the following is the metric system unit incorrectly paired with an abbreviation?
A) hectogram - hg
B) microgram - g
C) centimeter - hg
D) millimeter - mm
A) hectogram - hg
B) microgram - g
C) centimeter - hg
D) millimeter - mm
centimeter - hg
2
In which of the following is the metric system prefix incorrectly paired with a power of ten?
A) nano- and 10-9
B) micro- and 10-3
C) deca- and 101
D) giga- and 109
A) nano- and 10-9
B) micro- and 10-3
C) deca- and 101
D) giga- and 109
micro- and 10-3
3
In which of the following sequences are the metric system prefixes listed in order of decreasing size?
A) micro-, deca-, centi-
B) nano-, micro-, milli-
C) kilo-, centi-, nano-
D) hecto-, kilo-, deci-
A) micro-, deca-, centi-
B) nano-, micro-, milli-
C) kilo-, centi-, nano-
D) hecto-, kilo-, deci-
kilo-, centi-, nano-
4
What English unit is the closest distance to a metric kilometer?
A) yard
B) foot
C) inch
D) mile
A) yard
B) foot
C) inch
D) mile

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
The metric length unit that is closest in size to the English unit mile is ________.
A) meter
B) cubic centimeter
C) kilometer
D) megameter
A) meter
B) cubic centimeter
C) kilometer
D) megameter

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following measurements best estimates the length of a hardboiled egg?
A) 330 Km
B) 0.50 m
C) 50 mm
D) 11 dm
A) 330 Km
B) 0.50 m
C) 50 mm
D) 11 dm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
The coast guard seized 175 hectograms of cocaine on a Columbian boat they boarded off the coast of Florida. What is the mass of the cocaine seized in grams?
A) 1750 grams
B) 0.1759 grams
C) 17500 grams
D) 175000 grams
A) 1750 grams
B) 0.1759 grams
C) 17500 grams
D) 175000 grams

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following abbreviations stands for a unit of length?
A) mL
B) mg
C) cc
D) dm
A) mL
B) mg
C) cc
D) dm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the correct formula for calculating the volume of a sphere?
A) pr2h
B) pr3
C) 4/3 pr2h
D) 4/3 pr3
A) pr2h
B) pr3
C) 4/3 pr2h
D) 4/3 pr3

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following three-dimensional shapes is not correctly paired with the formula used to calculate its volume?
A) cube - s3
B) rectangular solid - l x w x h
C) cylinder - pr2h
D) sphere - pr3
A) cube - s3
B) rectangular solid - l x w x h
C) cylinder - pr2h
D) sphere - pr3

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
A decimeter equals:
A) 1 m
B) 1000 mm
C) 10 cm
D) 10 m
A) 1 m
B) 1000 mm
C) 10 cm
D) 10 m

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following units of measure is equivalent to a cm3 (cubic centimeter)?
A) mL
B) mm
C) mg
D) gr
A) mL
B) mm
C) mg
D) gr

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
A cylindrical slug of copper has a diameter of 68.3 mm and a height of 0.120 m. What is the volume of the cylinder of copper in cm3? (cylinder volume: V = pr2h)
A) 4.40 x 102 cm3
B) 702 cm3
C) 18.2 cm3
D) 176 cm3
A) 4.40 x 102 cm3
B) 702 cm3
C) 18.2 cm3
D) 176 cm3

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
In which of the following "unit set-ups" are the units incorrectly combined together?
A) dm4 x dm3 = dm7
B)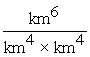 = km-2
= km-2
C)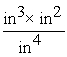 = in
= in
D)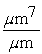 = ?m-2
= ?m-2
A) dm4 x dm3 = dm7
B)
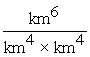 = km-2
= km-2C)
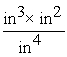 = in
= inD)
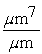 = ?m-2
= ?m-2
Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following unit conversion factor sequences would change milligrams to hectograms?
A) mg x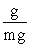 x
x 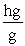
B) mg x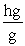 x
x 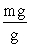
C) mg x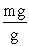 x
x 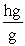
D) mg x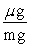 x
x 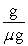
A) mg x
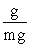 x
x 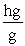
B) mg x
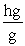 x
x 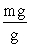
C) mg x
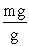 x
x 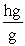
D) mg x
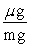 x
x 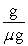

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements is false?
A) 2.5 x 107 milligrams is equivalent to 25 kilograms.
B) 6.7 cubic inches is equivalent to 1.1 x 105 cubic millimeters.
C) 9650 centigrams is equivalent to 0.213 pounds.
D) 0.250 hectometers is equivalent to 2.50 decimeters.
A) 2.5 x 107 milligrams is equivalent to 25 kilograms.
B) 6.7 cubic inches is equivalent to 1.1 x 105 cubic millimeters.
C) 9650 centigrams is equivalent to 0.213 pounds.
D) 0.250 hectometers is equivalent to 2.50 decimeters.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following unit conversion factors would change g/mL to lb/ft3 ?
A)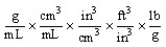
B)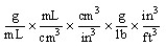
C)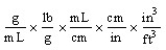
D)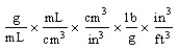
A)
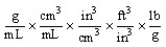
B)
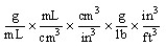
C)
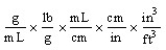
D)
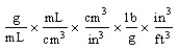

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following conversion factor sequences for units would effect the change from ft/sec to km/hr?
A)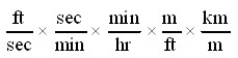
B)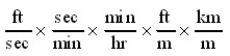
C)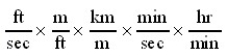
D)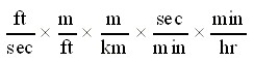
A)
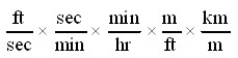
B)
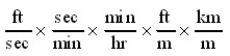
C)
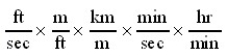
D)
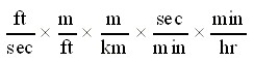

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is an incorrect conversion factor?
A)
B)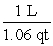
C)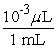
D)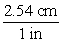
A)

B)
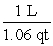
C)
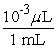
D)
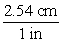

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
According to dimensional analysis, which of the following is the correct set-up for the problem "How many milligrams are there in 67 kilograms?"
A)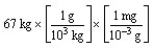
B)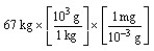
C)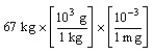
D)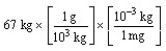
A)
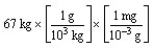
B)
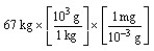
C)
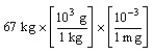
D)
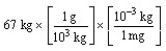

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
According to dimensional analysis, which of the following is a correct set-up for the problem "How many millimeters are there in 12.8 feet?"
A)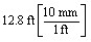
B)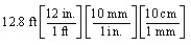
C)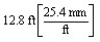
D)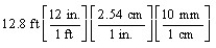
A)
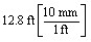
B)
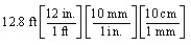
C)
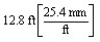
D)
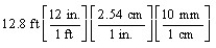

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
4.34 quarts is equivalent to how many microliters?
A) 4.11 x 103 mL
B) 4.11 x 106 mL
C) 6.74 x 105 mL
D) 2.69 mL
A) 4.11 x 103 mL
B) 4.11 x 106 mL
C) 6.74 x 105 mL
D) 2.69 mL

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
256 college students each donated one pint of blood. How many milliliters of blood did the students donate?
A) 121,100 mL
B) 6.84 x 106 mL
C) 3.89 x 10-8 mL
D) 16,574 mL
A) 121,100 mL
B) 6.84 x 106 mL
C) 3.89 x 10-8 mL
D) 16,574 mL

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
26.9 square centimeters is equivalent to how many square feet?
A) 117 ft2
B) 0.0290 ft2
C) 3.67 ft2
D) 0.00144 ft2
A) 117 ft2
B) 0.0290 ft2
C) 3.67 ft2
D) 0.00144 ft2

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following samples has the largest volume?
A) 3.2 x 103 dL
B) 3.2 x 10-4 kL
C) 3.2 x 10-4 hL
D) 3.2 x 10-6 L
A) 3.2 x 103 dL
B) 3.2 x 10-4 kL
C) 3.2 x 10-4 hL
D) 3.2 x 10-6 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
Which of these samples has the smallest mass?
A) 1.6 x 102 g
B) 1.6 x 10-2 g
C) 1.6 x 10-4 mg
D) 1.6 x 10-7 kg
A) 1.6 x 102 g
B) 1.6 x 10-2 g
C) 1.6 x 10-4 mg
D) 1.6 x 10-7 kg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
The typical volume of an aluminum can of soda is 355 mL. What is the equivalent volume in gallons?
A) 3.37 x 105 gal
B) 0.0939 gal
C) 5.75 gal
D) 0.673 gal
A) 3.37 x 105 gal
B) 0.0939 gal
C) 5.75 gal
D) 0.673 gal

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
The average adult has approximately 9.0 pints of blood. If 20 drops of blood is equivalent to 1 mL, how many drops of blood does the average human contain?
A) 85,000 drops
B) 1100 drops
C) 9.1 x 1011 drops
D) 230,000 drops
A) 85,000 drops
B) 1100 drops
C) 9.1 x 1011 drops
D) 230,000 drops

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
Global warming primarily results from the increasing concentration of carbon dioxide (CO2) in the Earth's atmosphere. A sport utility vehicle (SUV) typically produces 4.31 pounds of CO2 per gallon of gasoline consumed, is driven an average of 15,000 miles per year, and consumes gasoline at the rate of 14.2 miles per gallon. Calculate the annual production of CO2 (in pounds) by a typical SUV.
A) 26 lb/yr
B) 4600 lb/yr
C) 79 lb/yr
D) 980 lb/yr
A) 26 lb/yr
B) 4600 lb/yr
C) 79 lb/yr
D) 980 lb/yr

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
What is the mass of 36.0 mL of a liquid if its density is 2.70 g/mL?
A) 13.3 g
B) 0.0750 g
C) 97.2 g
D) 0.748 g
A) 13.3 g
B) 0.0750 g
C) 97.2 g
D) 0.748 g

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
What volume of a solution with a density of 13.9 g/mL is needed to provide 155 grams of solution?
A) 11.2 mL
B) 106 mL
C) 0.185 mL
D) 2150 mL
A) 11.2 mL
B) 106 mL
C) 0.185 mL
D) 2150 mL

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
If object A weighs 24 grams and has a volume of 3 mL and object B weighs 14 g and has a volume of 7 mL, ________.
A) B is more dense than A
B) A and B have equal densities
C) B is half as dense as A
D) A is four times as dense as B
A) B is more dense than A
B) A and B have equal densities
C) B is half as dense as A
D) A is four times as dense as B

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
Density is an easy way to distinguish between gold (density: approx. 19 g/cm3), fool's gold (density: approx. 4.5 g/cm3), and sand (density: approx. 2.5 g/cm3). If a large golden rock has a mass of 252 g and a volume of 56 cm3, which type of mineral is it?
A) gold
B) fool's gold
C) sand
D) none of these
A) gold
B) fool's gold
C) sand
D) none of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
Automotive batteries generally are filled with sulfuric acid. If a battery has a volume of 1.86 L and contains 3.42 x 103 grams of sulfuric acid, what is the density of sulfuric acid in g/mL?
A) 5.43 x 10-4 g/mL
B) 5.84 x 103 g/mL
C) 1.84 g/mL
D) 19.85 g/mL
A) 5.43 x 10-4 g/mL
B) 5.84 x 103 g/mL
C) 1.84 g/mL
D) 19.85 g/mL

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Chloroform was used as an anesthetic in the early days of surgery. If its density is 1.492 g/mL, what is the mass of 225 mL?
A) 336 g
B) 151 g
C) 225 g
D) 1.6 g
A) 336 g
B) 151 g
C) 225 g
D) 1.6 g

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
A sample of metal has a mass of 27.24 g and a volume of 2.20 mL. What is the correct value (correct number of significant figures) of its density using these data?
A) 12.382 g/mL
B) 0.081 g/mL
C) 12.4 g/mL
D) 0.0876 g/mL
A) 12.382 g/mL
B) 0.081 g/mL
C) 12.4 g/mL
D) 0.0876 g/mL

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
A certain children's fever reducing medicine has a concentration of 250 mg/10 mL. If a child is to receive 2 teaspoons of this medicine, how many mg of medicine is being received in one dose? (1 tsp = 5 mL)
A) 650 mg
B) 275 mg
C) 188 mg
D) 250 mg
A) 650 mg
B) 275 mg
C) 188 mg
D) 250 mg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
A certain infant analgesic medicine has a single dose of 36.0 mg/kg of body weight. How much analgesic will be administered in a single dose for an 18.4 pound toddler?
A) 436 mg
B) 80.1 mg
C) 3.00 x 102 mg
D) 921,000 mg
A) 436 mg
B) 80.1 mg
C) 3.00 x 102 mg
D) 921,000 mg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
What mass, in grams, of a 5.00% by mass sugar water solution is needed to supply 12.5 g of sugar?
A) 125 g
B) 2.50 x 102 g
C) 13.20 x 102 g
D) 150 g
A) 125 g
B) 2.50 x 102 g
C) 13.20 x 102 g
D) 150 g

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
How many grams of alcohol are present in 2 bottles (992 g total mass of mixture) of an alcohol/water mixture of that is 25.6% alcohol by mass?
A) 254 g alcohol
B) 366 g alcohol
C) 350 g alcohol
D) 210 g alcohol
A) 254 g alcohol
B) 366 g alcohol
C) 350 g alcohol
D) 210 g alcohol

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
In a laboratory class with 80 students, 50% of the students are male, and 10% of the male students are left handed. How many students in the laboratory are male students that are left handed?
A) 3 students
B) 8 students
C) 10 students
D) 4 students
A) 3 students
B) 8 students
C) 10 students
D) 4 students

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
Half-life is the time required for 50% of the compound to decay. If a 128 mg sample undergoes 6 successive half-lives, how much sample will be left?
A) 32.0 mg
B) 16.0 mg
C) 4.0 mg
D) 2.0 mg
A) 32.0 mg
B) 16.0 mg
C) 4.0 mg
D) 2.0 mg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
The accepted value for the boiling point of diethylether is 35 °C. What is the percent error for a reported value of 36.1 °C as the boiling point of diethylether?
A) -3.1%
B) -8.6%
C) 5.7%
D) 3.1%
A) -3.1%
B) -8.6%
C) 5.7%
D) 3.1%

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
A student was to determine the calibration error of a 100 mL graduated cylinder. Water at 25.0 °C was added to the cylinder until the level reached 100.0 mL. Knowing the mass of water in the cylinder and the density of water at 25.0 °C, the actual volume of water in the cylinder was calculated to be 99.2 mL. Calculate the percent error in the calibration of the graduated cylinder.
A) 0.8%
B) 2%
C) -0.8%
D) 1%
A) 0.8%
B) 2%
C) -0.8%
D) 1%

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
A student was asked to determine the density of magnesium metal by the volume of displacement method. The student determined the density of magnesium as 1.89 g/mL. The accepted value for the density of magnesium is 1.74 g/mL. Calculate the percent error associated with the student's reported density.
A) 8.6%
B) 7.9%
C) 92%
D) 86%
A) 8.6%
B) 7.9%
C) 92%
D) 86%

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following statements concerning the three major temperature scales is incorrect?
A) Kelvin temperatures always have positive values.
B) The freezing point of water has a lower numerical value on the Kelvin scale than on the Fahrenheit scale.
C) The boiling point of water has a higher numerical value on the Fahrenheit scale than the Celsius scale.
D) The boiling point of water has a higher numerical value on the Kelvin scale than the Celsius scale.
A) Kelvin temperatures always have positive values.
B) The freezing point of water has a lower numerical value on the Kelvin scale than on the Fahrenheit scale.
C) The boiling point of water has a higher numerical value on the Fahrenheit scale than the Celsius scale.
D) The boiling point of water has a higher numerical value on the Kelvin scale than the Celsius scale.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
A temperature change of 16.0 degrees Fahrenheit is equivalent to how many degrees change on the Celsius temperature scale?
A) 8.89°
B) 18.7°
C) 2.56°
D) 5.76°
A) 8.89°
B) 18.7°
C) 2.56°
D) 5.76°

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
A temperature of 316° on the Kelvin scale is equivalent to ________.
A) 589 °C
B) 589 °C.
C) 43 °C
D) 104 °C
A) 589 °C
B) 589 °C.
C) 43 °C
D) 104 °C

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
If the temperature is 18 °C then the reading on the Fahrenheit scale would be ________.
A) 40 °F
B) 64 °F
C) 38 °F
D) 12 °F
A) 40 °F
B) 64 °F
C) 38 °F
D) 12 °F

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
The surface temperature of the sun is 7530 °C. What is the surface temperature of the sun in degrees Kelvins?
A) 10,400 K
B) 273 K
C) 420 K
D) 7803 K
A) 10,400 K
B) 273 K
C) 420 K
D) 7803 K

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
The temperature of an average lightning bolt is 3.0 x 104 °C. What is the equivalent temperature on the Fahrenheit scale?
A) 5.4 x 104 °F
B) 6.9 x 105 °F
C) 1.7 x 104 °F
D) 3.8 x 103 °F
A) 5.4 x 104 °F
B) 6.9 x 105 °F
C) 1.7 x 104 °F
D) 3.8 x 103 °F

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
An astronaut whose mass is equal to 82 kg on Earth, on the Moon will
-weigh much less due to the moon's decreased gravitational attraction.
-weigh much less due to the moon's decreased gravitational attraction.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
An astronaut whose mass is equal to 82 kg on Earth, on the Moon will
-weigh much more due to the Moon's decreased gravitational attraction.
-weigh much more due to the Moon's decreased gravitational attraction.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
An astronaut whose mass is equal to 82 kg on Earth, on the Moon will
-have the same mass on the Moon as on Earth.
-have the same mass on the Moon as on Earth.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
The density of osmium, the densest metal known, is 2260 cg/cm3. A rectangular block of osmium is depicted below. Calculate the mass of the block of osmium in pounds.
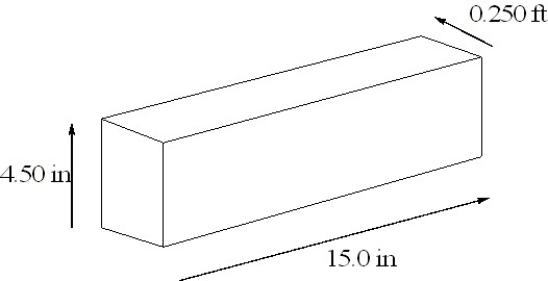


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate the volume of the sphere depicted below in cubic centimeters.
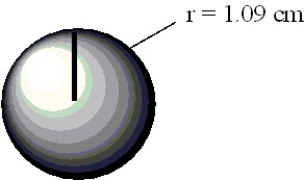


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
Using the dimensional analysis method of problem solving, carry out the following metric-metric conversions:
-17.2 cm = ? m
-17.2 cm = ? m

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
Using the dimensional analysis method of problem solving, carry out the following metric-metric conversions:
-1.786 kg = ? μg
-1.786 kg = ? μg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
Using the dimensional analysis method of problem solving, carry out the following metric-metric conversions:
-257 mL = ? dL
-257 mL = ? dL

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
Using the dimensional analysis method of problem solving, carry out the following metric-metric conversions:
-65 cm3 = ? km3
-65 cm3 = ? km3

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
Using the dimensional analysis method of problem solving, carry out the following metric-metric conversions:
-3,634 mg = ? hg
-3,634 mg = ? hg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
Using the dimensional analysis method of problem solving, carry out the following English-metric or metric-English conversions:
-25.2 lb = ? mg
-25.2 lb = ? mg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
Using the dimensional analysis method of problem solving, carry out the following English-metric or metric-English conversions:
-4.2 gal = ? cL
-4.2 gal = ? cL

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
Using the dimensional analysis method of problem solving, carry out the following English-metric or metric-English conversions:
-3.3 m = ? ft
-3.3 m = ? ft

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
Using the dimensional analysis method of problem solving, carry out the following English-metric or metric-English conversions:
-2.7 cm2 = ? in2
-2.7 cm2 = ? in2

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
Using the dimensional analysis method of problem solving, carry out the following English-metric or metric-English conversions:
-0.650 mile = ? cm
-0.650 mile = ? cm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
Using the dimensional analysis method of problem solving, calculate the velocity, in yards per second, that is equivalent to a velocity of 50.00 m/day.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
A post-operative patient is to receive 250 mg of intravenous (IV) antibiotic every 4.00 hours. A 1.00 liter bag of IV solution contains 1000 mg of antibiotic. What drop rate (in drops per second) of IV solution is required for the patient to receive the correct dosage of antibiotic? (20 drops = 1 mL)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
During the summertime in Atlanta, the jet stream moves north towards Canada, and the atmosphere often stagnates. Weather forecasters give daily ozone warnings to the elderly and to people with asthma because ozone build-up in the air is harmful. Typically, an adult will inhale 2.1 liters of air per breath. What mass of ozone, in g, is taken into the lungs per breath by an adult breathing air containing 3.64 10-4 g of ozone per cubic centimeter (cm3) of air?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
What is the capacity of a gasoline container, in gallons, if it will hold 96.5 lb of gasoline of density 0.60 g/mL?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
If the speed of a bullet being fired from a rifle is 2357 ft/sec, what is this in miles per hour?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
The average person requires 2500 Calories per day to maintain normal body function. Generally speaking, an average person gains one pound of body weight for every 3500 Calories consumed beyond that required by the body. If an average person's diet is 2700 Calories per day for one year, how many pounds of body weight would the person gain during that year?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
The cheetah is the fastest land animal on earth with a top speed of 70.0 miles per hour. What is the top speed of a cheetah in meters per second?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
The concentration of sugar in a solution is found to be 2.30 g/L. What is the sugar concentration in g/cm3?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
What mass of gold (density = 19.3 g/cm3) occupies the same volume as 80.0 g of lithium (density = 0.534 g/cm3)?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
Using the dimensional analysis method of problem solving, calculate the number of green brass buttons in a box containing 1365 buttons, if 36.2% of the buttons are brass and 18.1% of the brass buttons are green.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
A temperature of 354 on the Kelvin scale is equivalent to what temperature on the Fahrenheit scale?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
A hospital patient has an oral temperature of 39.5 °C and weighs 190. lb. He is to receive a drug, the total dosage of which is 55 mg per kilogram of body weight. The drug is dissolved in water (25 mg per milliliter).
-What is his temperature in degrees Fahrenheit? In Kelvin?
-What is his temperature in degrees Fahrenheit? In Kelvin?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
A hospital patient has an oral temperature of 39.5 °C and weighs 190. lb. He is to receive a drug, the total dosage of which is 55 mg per kilogram of body weight. The drug is dissolved in water (25 mg per milliliter).
-What is his weight in kilograms?
-What is his weight in kilograms?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
A hospital patient has an oral temperature of 39.5 °C and weighs 190. lb. He is to receive a drug, the total dosage of which is 55 mg per kilogram of body weight. The drug is dissolved in water (25 mg per milliliter).
-What mass in milligrams of pure drug should he receive?
-What mass in milligrams of pure drug should he receive?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


