Exam 3: Unit Systems and Dimensional Analysis
Exam 1: The Science of Chemistry62 Questions
Exam 2: Numbers From Measurements99 Questions
Exam 3: Unit Systems and Dimensional Analysis83 Questions
Exam 4: Basic Concepts About Matter75 Questions
Exam 5: Atoms, Molecules, Formulas, and Subatomic Particles87 Questions
Exam 6: Electronic Structure and Chemical Periodicity92 Questions
Exam 7: Chemical Bonds92 Questions
Exam 8: Chemical Nomenclature97 Questions
Exam 9: Chemical Calculations: the Mole Concept and Chemical Formulas93 Questions
Exam 10: Chemical Calculations Involving Chemical Equations68 Questions
Exam 11: States of Matter65 Questions
Exam 12: Gas Laws78 Questions
Exam 13: Solutions64 Questions
Exam 14: Acids, Bases and Salts65 Questions
Exam 15: Oxidation and Reduction70 Questions
Exam 16: Reaction Rates and Chemical Equilibrium52 Questions
Exam 17: Nuclear Chemistry69 Questions
Select questions type
Which of the following is an incorrect conversion factor?
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
C
During the summertime in Atlanta, the jet stream moves north towards Canada, and the atmosphere often stagnates. Weather forecasters give daily ozone warnings to the elderly and to people with asthma because ozone build-up in the air is harmful. Typically, an adult will inhale 2.1 liters of air per breath. What mass of ozone, in g, is taken into the lungs per breath by an adult breathing air containing 3.64 10-4 g of ozone per cubic centimeter (cm3) of air?
Free
(Essay)
5.0/5  (40)
(40)
Correct Answer:
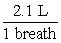 x
x 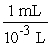 x
x 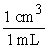 x
x 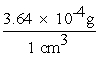 = 0.7644 g/breath (calc) = 0.76 g/breath (corr)
= 0.7644 g/breath (calc) = 0.76 g/breath (corr)
According to dimensional analysis, which of the following is a correct set-up for the problem "How many millimeters are there in 12.8 feet?"
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
D
A student was asked to determine the density of magnesium metal by the volume of displacement method. The student determined the density of magnesium as 1.89 g/mL. The accepted value for the density of magnesium is 1.74 g/mL. Calculate the percent error associated with the student's reported density.
(Multiple Choice)
4.8/5  (41)
(41)
What English unit is the closest distance to a metric kilometer?
(Multiple Choice)
4.9/5  (34)
(34)
Using the dimensional analysis method of problem solving, carry out the following English-metric or metric-English conversions:
-3.3 m = ? ft
(Essay)
4.9/5  (43)
(43)
Methane is a natural gas that is used in most homes. If it boils at 111 K what is this boiling point in Celsius and Farenheit?
(Short Answer)
4.7/5  (35)
(35)
If the speed of a bullet being fired from a rifle is 2357 ft/sec, what is this in miles per hour?
(Short Answer)
4.8/5  (35)
(35)
Using the dimensional analysis method of problem solving, carry out the following English-metric or metric-English conversions:
-4.2 gal = ? cL
(Essay)
4.9/5  (37)
(37)
The surface temperature of the sun is 7530 °C. What is the surface temperature of the sun in degrees Kelvins?
(Multiple Choice)
4.9/5  (33)
(33)
The recommended adult dose of Elixophyllin, a drug used to treat asthma, is 9.0 mg/kg of body mass. Calculate the dose in milligrams for a 185-lb. person.
(Short Answer)
4.7/5  (34)
(34)
Calculate the volume of the sphere depicted below in cubic centimeters.
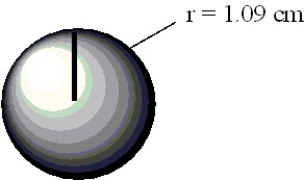
(Essay)
4.9/5  (41)
(41)
26.9 square centimeters is equivalent to how many square feet?
(Multiple Choice)
4.8/5  (42)
(42)
A hospital patient has an oral temperature of 39.5 °C and weighs 190. lb. He is to receive a drug, the total dosage of which is 55 mg per kilogram of body weight. The drug is dissolved in water (25 mg per milliliter).
-What is his temperature in degrees Fahrenheit? In Kelvin?
(Short Answer)
4.9/5  (42)
(42)
Which of the following conversion factor sequences for units would effect the change from ft/sec to km/hr?
(Multiple Choice)
4.8/5  (40)
(40)
Global warming primarily results from the increasing concentration of carbon dioxide (CO2) in the Earth's atmosphere. A sport utility vehicle (SUV) typically produces 4.31 pounds of CO2 per gallon of gasoline consumed, is driven an average of 15,000 miles per year, and consumes gasoline at the rate of 14.2 miles per gallon. Calculate the annual production of CO2 (in pounds) by a typical SUV.
(Multiple Choice)
4.9/5  (45)
(45)
A student was to determine the calibration error of a 100 mL graduated cylinder. Water at 25.0 °C was added to the cylinder until the level reached 100.0 mL. Knowing the mass of water in the cylinder and the density of water at 25.0 °C, the actual volume of water in the cylinder was calculated to be 99.2 mL. Calculate the percent error in the calibration of the graduated cylinder.
(Multiple Choice)
4.9/5  (40)
(40)
A temperature of 354 on the Kelvin scale is equivalent to what temperature on the Fahrenheit scale?
(Essay)
4.9/5  (41)
(41)
Showing 1 - 20 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)