Deck 2: Alkanes and Cycloakanes: Introduction to Hydrocarbons
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/18
Play
Full screen (f)
Deck 2: Alkanes and Cycloakanes: Introduction to Hydrocarbons
1
Alkanes are characterized by the general molecular formula:
A)
B)
C)
D)
A)
B)
C)
D)
2
Cycloalkanes are characterized by the general molecular formula:
A)
B)
C)
D)
A)
B)
C)
D)
3
The carbon-carbon sigma bond in ethane is formed by overlap of which two orbitals?
A)
B)
C)
D)
A)
B)
C)
D)
4
What is the IUPAC name of the following?
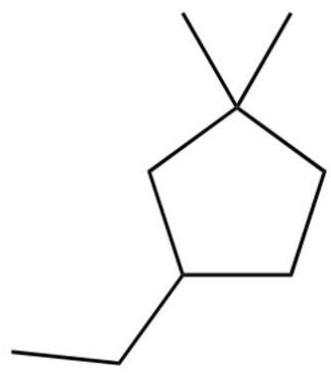
A) 1-ethyl-4.4-dimethylcyclopentane
B) 1-ethyl-3, 3-dimethylcyclopentane
C) 3-ethyl-1, 1-dimethylcyclopentane
D) 4-ethyl-1, 1-dimethylcyclopentane

A) 1-ethyl-4.4-dimethylcyclopentane
B) 1-ethyl-3, 3-dimethylcyclopentane
C) 3-ethyl-1, 1-dimethylcyclopentane
D) 4-ethyl-1, 1-dimethylcyclopentane

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
5
The sigma bond in acetylene is formed by the overlap of which two orbitals?
A)
B)
C)
D)
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following are constitutional isomers?
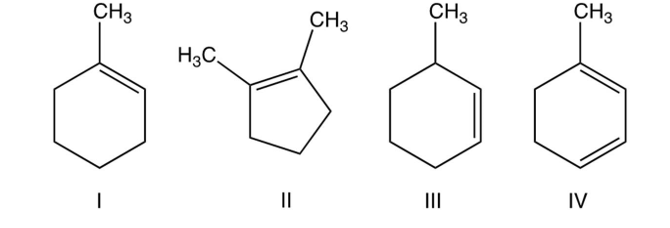
A) I, II, and III
B) I, III, and IV
C) only I and III
D) all are constitutional isomers.

A) I, II, and III
B) I, III, and IV
C) only I and III
D) all are constitutional isomers.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
7
Arrange the following isomeric alkanes in order of increasing boiling point.
I. -heptane
II. 2,3-dimethylpentane
III. 2,2,3-trimethylbutane
A) I II III
B) II III I
C) III I II
D) III II I
I. -heptane
II. 2,3-dimethylpentane
III. 2,2,3-trimethylbutane
A) I II III
B) II III I
C) III I II
D) III II I

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following has(have) a higher oxidation state of carbon than the carbon in formaldehyde, ?
I.
II.
III.
A) I
B) III
C) II and III
D) I, II, and III
I.
II.
III.
A) I
B) III
C) II and III
D) I, II, and III

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
9
Carbon atoms 1,2 , and 3 in the following structure are classified, respectively, as
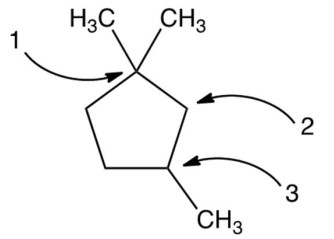
A) tertiary, primary, secondary.
B) quaternary, primary, tertiary.
C) quaternary, secondary, secondary.
D) quaternary, secondary, tertiary.

A) tertiary, primary, secondary.
B) quaternary, primary, tertiary.
C) quaternary, secondary, secondary.
D) quaternary, secondary, tertiary.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
10
The heats of combustion of heptane and 3,3-dimethypentane are 4,817 and , respectively. Which statement is true?
A) Heptane is more stable then 3,3-dimethylpentane.
B) 3,3-Dimethylpentane is more stable than heptane.
C) Stabilities cannot be compared since they are not isomers.
D) Stabilities cannot be compared since they give different combustion products.
A) Heptane is more stable then 3,3-dimethylpentane.
B) 3,3-Dimethylpentane is more stable than heptane.
C) Stabilities cannot be compared since they are not isomers.
D) Stabilities cannot be compared since they give different combustion products.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
11
The reaction of acetylene with hydrogen gas is shown below. Which statements are true concerning the reaction?
I. Acetylene is oxidized to ethane.
II. Acetylene is reduced to ethane.
III. Carbon changes oxidation state from -1 to -3 .
IV. Hydrogen (from ) changes oxidation state from 0 to +1 .
A) I and III
B) II and IV
C) I, III, and IV
D) II, III, and IV
I. Acetylene is oxidized to ethane.
II. Acetylene is reduced to ethane.
III. Carbon changes oxidation state from -1 to -3 .
IV. Hydrogen (from ) changes oxidation state from 0 to +1 .
A) I and III
B) II and IV
C) I, III, and IV
D) II, III, and IV

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
12
How many moles of gas would be consumed in the complete combustion of 0.100 mole of ?
A) 0.100 mole
B) 0.400 mole
C) 0.800 mole
D)
A) 0.100 mole
B) 0.400 mole
C) 0.800 mole
D)

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
13
What is the relationship between the two structures below?

A) identical structures
B) resonance forms
C) constitutional isomers
D) different compounds with different compositions

A) identical structures
B) resonance forms
C) constitutional isomers
D) different compounds with different compositions

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
14
What is the IUPAC name of the following structure?
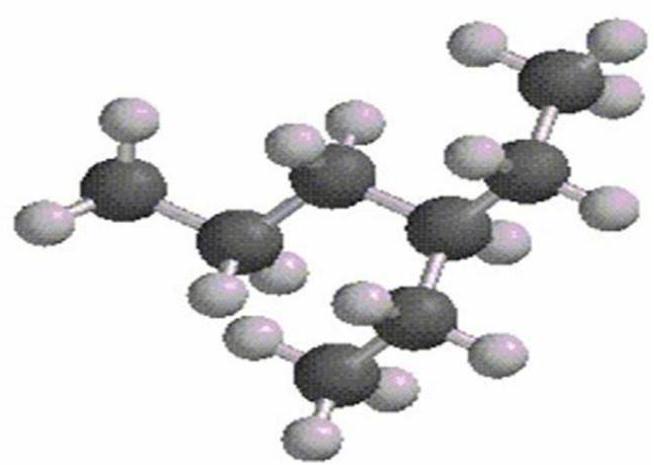
A) 3-propylpentane
B) 3-ethylhexane
C) 2-ethylheptane
D) 4-ethylpentane

A) 3-propylpentane
B) 3-ethylhexane
C) 2-ethylheptane
D) 4-ethylpentane

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
15
What is the estimated bond angle in the following structure?
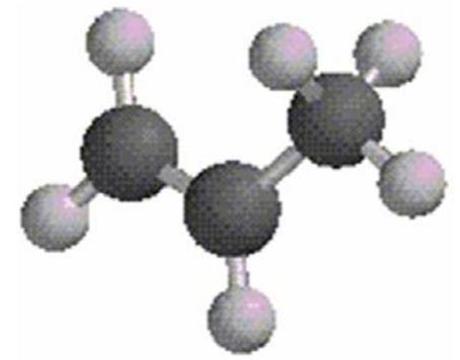
A)
B)
C)
D)

A)
B)
C)
D)

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
16
The orbitals of carbon in are formed from the
A) three orbitals.
B) and one of the orbitals.
C) and two of the orbitals.
D) and the three orbitals.
A) three orbitals.
B) and one of the orbitals.
C) and two of the orbitals.
D) and the three orbitals.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
17
What is the bond angle in ?
A)
B)
C)
D)
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
18
The carbon-carbon single bond in the following is formed by the overlap of which two orbitals?
A)
B)
C)
D)
A)
B)
C)
D)

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck


