Exam 2: Alkanes and Cycloakanes: Introduction to Hydrocarbons
Exam 1: Structure Determines Properties15 Questions
Exam 2: Alkanes and Cycloakanes: Introduction to Hydrocarbons18 Questions
Exam 3: Alkanes and Cycloalkanes: Introduction to Stereochemistry13 Questions
Exam 4: Alcohols and Alkyl Halides: Introduction to Reaction Mechanisms11 Questions
Exam 5: Structure and Preparation of Alkenes: Elimination Reactions18 Questions
Exam 6: Addition Reactions of Alkenes13 Questions
Exam 7: Chirality12 Questions
Exam 8: Nucleophilic Substitution14 Questions
Exam 9: Alkynes15 Questions
Exam 13: Spectroscopy15 Questions
Exam 14: Organometallic Compounds13 Questions
Exam 15: Alcohols, Diols, and Thiols15 Questions
Exam 16: Ethers, Epoxides, and Sulfides13 Questions
Exam 17: Aldehydes and Ketones: Nucleophilic Addition to the Carbonyl Group15 Questions
Exam 18: Carboxylic Acids10 Questions
Exam 19: Acid Derivatives17 Questions
Exam 20: Enols and Enolates14 Questions
Exam 21: Amines12 Questions
Exam 22: Phenols11 Questions
Exam 23: Carbohydrates15 Questions
Exam 24: Lipids13 Questions
Exam 25: Amino Acids, Peptides, and Proteins16 Questions
Exam 26: Nucleosides, Nucleotides, and Nucleic Acids15 Questions
Exam 27: Synthetic Polymers12 Questions
Select questions type
The reaction of acetylene with hydrogen gas is shown below. Which statements are true concerning the reaction?
I. Acetylene is oxidized to ethane.
II. Acetylene is reduced to ethane.
III. Carbon changes oxidation state from -1 to -3 .
IV. Hydrogen (from ) changes oxidation state from 0 to +1 .
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
D
What is the IUPAC name of the following?
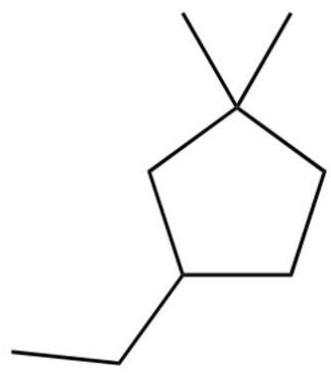
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
C
Alkanes are characterized by the general molecular formula:
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
C
The sigma bond in acetylene is formed by the overlap of which two orbitals?
(Multiple Choice)
4.7/5  (41)
(41)
Carbon atoms 1,2 , and 3 in the following structure are classified, respectively, as
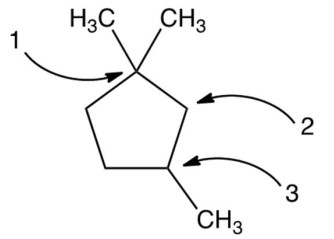
(Multiple Choice)
4.8/5  (37)
(37)
What is the estimated bond angle in the following structure?
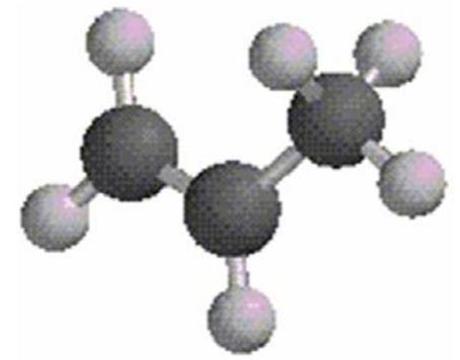
(Multiple Choice)
4.8/5  (37)
(37)
Arrange the following isomeric alkanes in order of increasing boiling point.
I. -heptane
II. 2,3-dimethylpentane
III. 2,2,3-trimethylbutane
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following has(have) a higher oxidation state of carbon than the carbon in formaldehyde, ?
I.
II.
III.
(Multiple Choice)
4.9/5  (38)
(38)
The carbon-carbon sigma bond in ethane is formed by overlap of which two orbitals?
(Multiple Choice)
4.9/5  (43)
(43)
The heats of combustion of heptane and 3,3-dimethypentane are 4,817 and , respectively. Which statement is true?
(Multiple Choice)
4.8/5  (38)
(38)
How many moles of gas would be consumed in the complete combustion of 0.100 mole of ?
(Multiple Choice)
4.8/5  (37)
(37)
Cycloalkanes are characterized by the general molecular formula:
(Multiple Choice)
4.8/5  (40)
(40)
The carbon-carbon single bond in the following is formed by the overlap of which two orbitals?
(Multiple Choice)
4.7/5  (36)
(36)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)