Deck 4: Alcohols and Alkyl Halides: Introduction to Reaction Mechanisms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/11
Play
Full screen (f)
Deck 4: Alcohols and Alkyl Halides: Introduction to Reaction Mechanisms
1
What is the IUPAC name of the compound below?
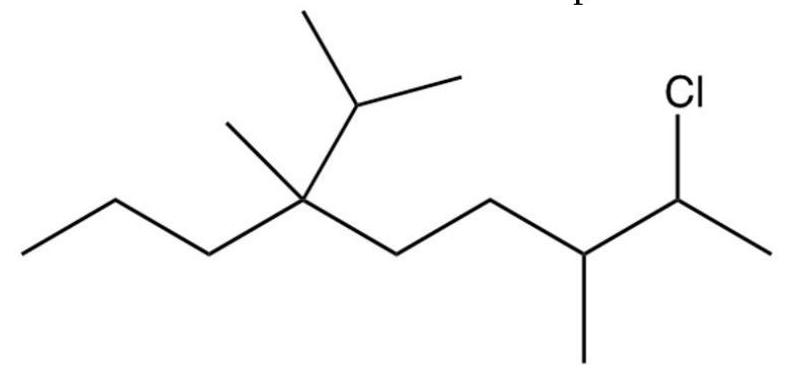
A) 8-chloro-4-isopropyl-4,7-dimethylnonane
B) 2-chloro-6-isopropyl-3,6-dimethylnonane
C) 2-chloro-3,6,7-trimethyl-6-propyloctane
D) 6-sec-butyl-2-chloro-3,6-dimethyloctane

A) 8-chloro-4-isopropyl-4,7-dimethylnonane
B) 2-chloro-6-isopropyl-3,6-dimethylnonane
C) 2-chloro-3,6,7-trimethyl-6-propyloctane
D) 6-sec-butyl-2-chloro-3,6-dimethyloctane
2-chloro-6-isopropyl-3,6-dimethylnonane
2
Chlorination of pentane gives a mixture of isomers having the molecular formula . The percentage of 1 -chloropentane is . Assuming the secondary hydrogens in pentane are equally reactive to monochlorination, what is the percentage of 3-chloropentane in the mixture?
A)
B)
C)
D)
A)
B)
C)
D)
3
Which one of the following gives a single monochlorination product?
A) 2,2-dimethylpropane
B) 2,2-dimethylbutane
C) 2,3-dimethylbutane
D) 2-methylpropane
A) 2,2-dimethylpropane
B) 2,2-dimethylbutane
C) 2,3-dimethylbutane
D) 2-methylpropane
2,2-dimethylpropane
4
What are the products of the following reaction?
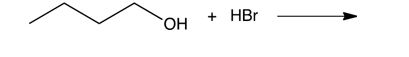
A) 1-bromobutane and water
B) 1-bromobutane and hydrogen
C) butane and
D) hydrogen
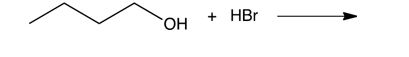
A) 1-bromobutane and water
B) 1-bromobutane and hydrogen
C) butane and
D) hydrogen

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
5
Arrange the following alcohols in order of their decreasing reactivity with (most reactive first).
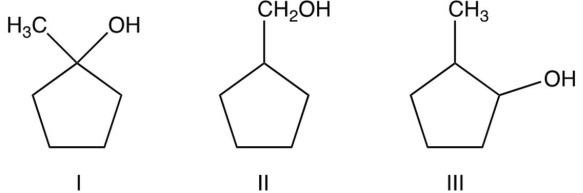
A) I II III
B) I III II
C) III I II
D) II III I

A) I II III
B) I III II
C) III I II
D) II III I

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
6
Arrange the following carbocations in order of their decreasing stabilities (most stable first).
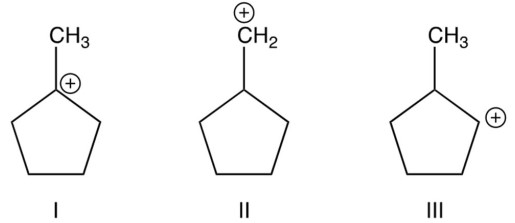
A) I II III
B) III II I
C) I III II
D) II III I

A) I II III
B) III II I
C) I III II
D) II III I

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the most stable radical?
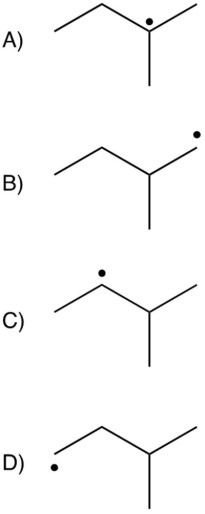
A)
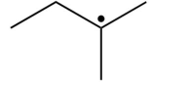
B)
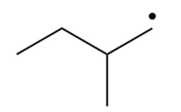
C)
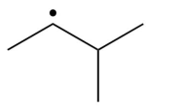
D)
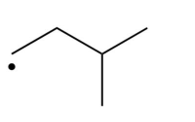

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
8
The central carbon of the tert-butyl carbocation, , is
A) hybridized with formal charge.
B) hybridized with a 0 formal charge.
C) hybridized with formal charge.
D) hybridized with a 0 formal charge.
A) hybridized with formal charge.
B) hybridized with a 0 formal charge.
C) hybridized with formal charge.
D) hybridized with a 0 formal charge.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
9
Dibromination of isopropylcyclopentane gives a product which can be isolated in good yields. Which of the following would you predict to be this product?
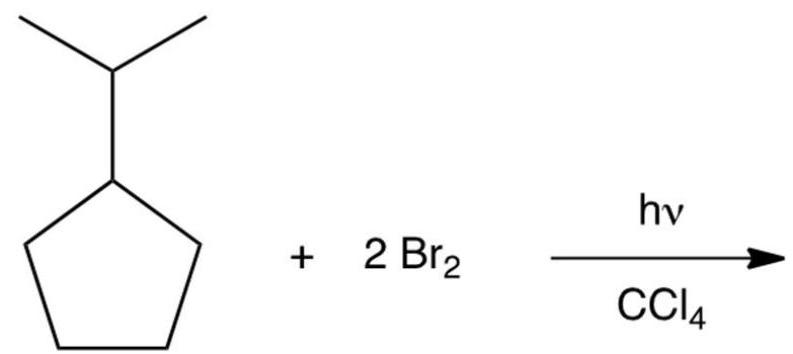
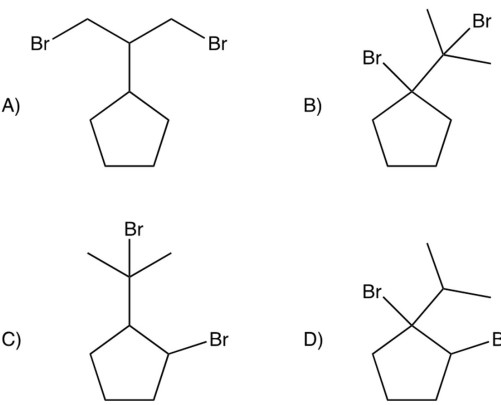
A) A
B) B
C) C
D) D
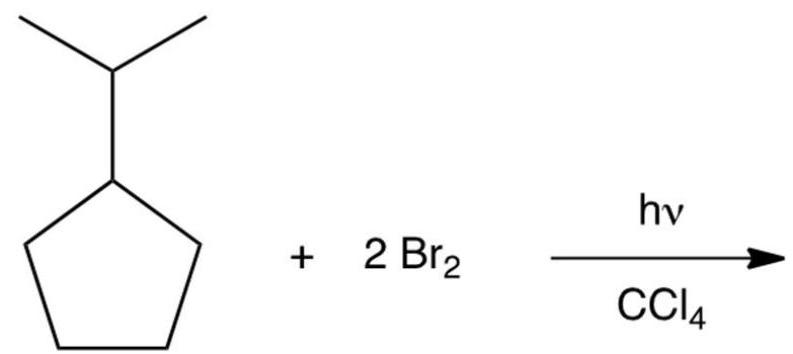

A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following mechanistically depicts the protonation of tert-butyl alcohol by hydrogen bromide?
A)
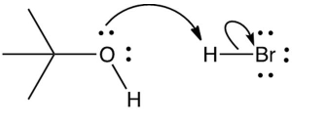
B)
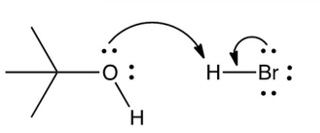
C)
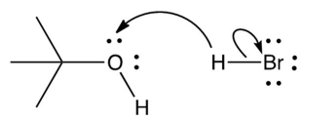
D)
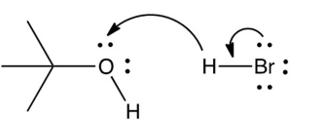
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
11
If the compound below containing three types of alcohols were exposed to ONLY 1 equivalent of , what major product would you expect?
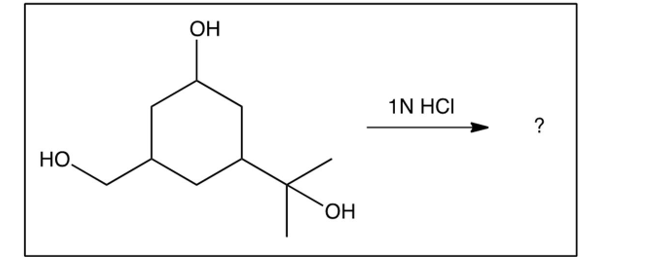
A)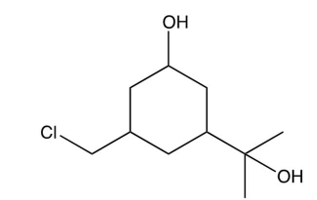
B)
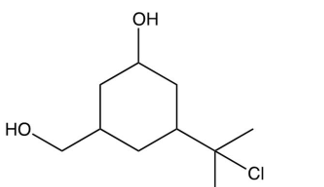
C)
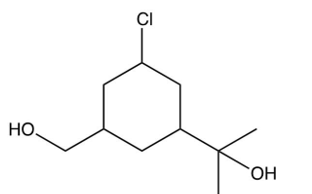
D) there is no way to know

A)

B)

C)

D) there is no way to know

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck


