Exam 4: Alcohols and Alkyl Halides: Introduction to Reaction Mechanisms
Exam 1: Structure Determines Properties15 Questions
Exam 2: Alkanes and Cycloakanes: Introduction to Hydrocarbons18 Questions
Exam 3: Alkanes and Cycloalkanes: Introduction to Stereochemistry13 Questions
Exam 4: Alcohols and Alkyl Halides: Introduction to Reaction Mechanisms11 Questions
Exam 5: Structure and Preparation of Alkenes: Elimination Reactions18 Questions
Exam 6: Addition Reactions of Alkenes13 Questions
Exam 7: Chirality12 Questions
Exam 8: Nucleophilic Substitution14 Questions
Exam 9: Alkynes15 Questions
Exam 13: Spectroscopy15 Questions
Exam 14: Organometallic Compounds13 Questions
Exam 15: Alcohols, Diols, and Thiols15 Questions
Exam 16: Ethers, Epoxides, and Sulfides13 Questions
Exam 17: Aldehydes and Ketones: Nucleophilic Addition to the Carbonyl Group15 Questions
Exam 18: Carboxylic Acids10 Questions
Exam 19: Acid Derivatives17 Questions
Exam 20: Enols and Enolates14 Questions
Exam 21: Amines12 Questions
Exam 22: Phenols11 Questions
Exam 23: Carbohydrates15 Questions
Exam 24: Lipids13 Questions
Exam 25: Amino Acids, Peptides, and Proteins16 Questions
Exam 26: Nucleosides, Nucleotides, and Nucleic Acids15 Questions
Exam 27: Synthetic Polymers12 Questions
Select questions type
Which of the following mechanistically depicts the protonation of tert-butyl alcohol by hydrogen bromide?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
A
Dibromination of isopropylcyclopentane gives a product which can be isolated in good yields. Which of the following would you predict to be this product?
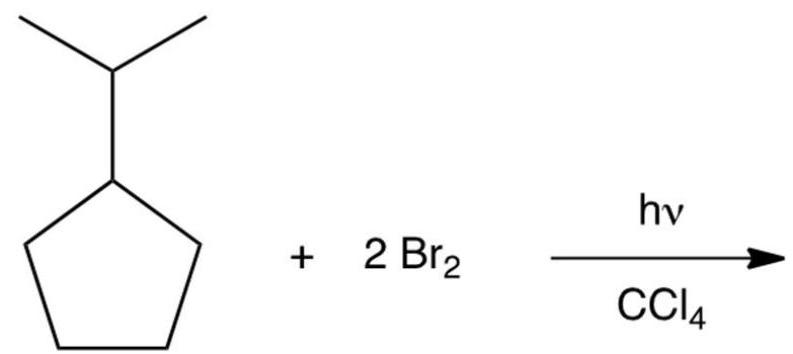
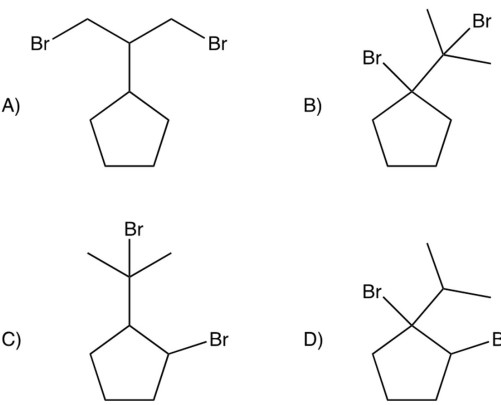
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
B
The central carbon of the tert-butyl carbocation, , is
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
A
If the compound below containing three types of alcohols were exposed to ONLY 1 equivalent of , what major product would you expect?
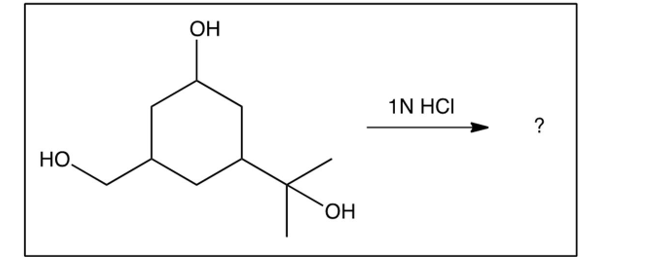
(Multiple Choice)
4.9/5  (30)
(30)
Which one of the following gives a single monochlorination product?
(Multiple Choice)
4.9/5  (34)
(34)
Chlorination of pentane gives a mixture of isomers having the molecular formula . The percentage of 1 -chloropentane is . Assuming the secondary hydrogens in pentane are equally reactive to monochlorination, what is the percentage of 3-chloropentane in the mixture?
(Multiple Choice)
4.8/5  (31)
(31)
Arrange the following carbocations in order of their decreasing stabilities (most stable first).
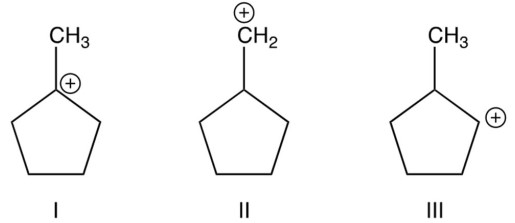
(Multiple Choice)
4.9/5  (35)
(35)
Arrange the following alcohols in order of their decreasing reactivity with (most reactive first).
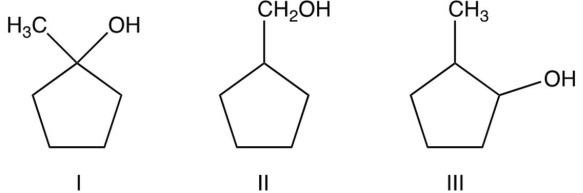
(Multiple Choice)
4.9/5  (42)
(42)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)