Deck 4: Quantum Mechanics, Atomic Structure, Quantum Mechanics and Molecular Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/9
Play
Full screen (f)
Deck 4: Quantum Mechanics, Atomic Structure, Quantum Mechanics and Molecular Structure
1
A MO orbital for a heteronuclear diatomic is given by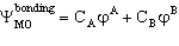
If A is more electronegative than B then you would predict that
A) CA > CB
B) CA = CB
C) CA < CB
D) CA = -CB
E) you would not expect any relation between these coefficients and the electronegativity of the elements A & B
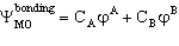
If A is more electronegative than B then you would predict that
A) CA > CB
B) CA = CB
C) CA < CB
D) CA = -CB
E) you would not expect any relation between these coefficients and the electronegativity of the elements A & B
CA > CB
2
The molecule HeH+ has an antibonding MO of the form
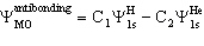
For this you would expect
A) C1 > C2
B) C2 < C2
C) C1 = C2
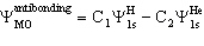
For this you would expect
A) C1 > C2
B) C2 < C2
C) C1 = C2
C1 > C2
3
Oxygen is paramagnetic and has a bond order of two. Which of the following represents the ground electronic state for oxygen?
A)
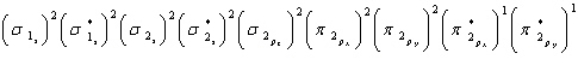
B)
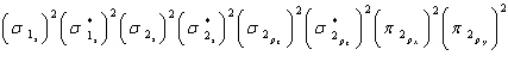
C)
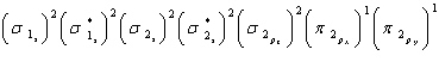
D) A and C are equally probable
E) None of the above
A)
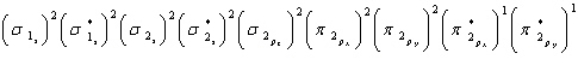
B)
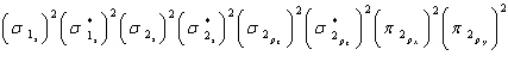
C)
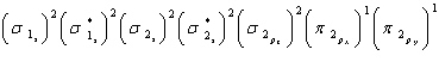
D) A and C are equally probable
E) None of the above
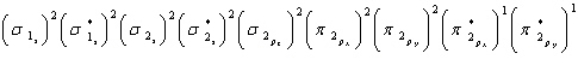
4
Which of the following combinations of atomic orbitals will result in a bonding molecular orbital for a homonuclear diatomic molecule when the z-axis is aligned with the nuclei of the bonded atom?
A)
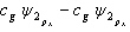
B)
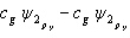
C)
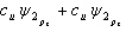
D)
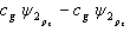
E) A, B, and D
A)
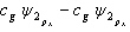
B)
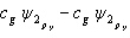
C)
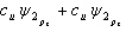
D)
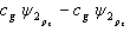
E) A, B, and D

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
5
Given that Zeff(2s) in Li is 1.26, estimate the energy of the 2s electron in Li in Rhydberg units.
A) -0.40
B) -0.63
C) -0.79
D) -1.26
E) -4.0
A) -0.40
B) -0.63
C) -0.79
D) -1.26
E) -4.0

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
6
Rank from smallest to largest in terms of atom/ionic radii.
A) S2-, Cl-, Ar, K+, Ca2+
B) Ca2+, K+, Ar, Cl-, S2-
C) Ca2+, S2-, K+, Cl-, Ar
D) Ar, K+, Cl-, Ca2+, S2-
E) Ar, Cl-, K+, S2-, Ca2+
A) S2-, Cl-, Ar, K+, Ca2+
B) Ca2+, K+, Ar, Cl-, S2-
C) Ca2+, S2-, K+, Cl-, Ar
D) Ar, K+, Cl-, Ca2+, S2-
E) Ar, Cl-, K+, S2-, Ca2+

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
7
What do you predict of the electron configuration of element 117?
A) [Rn]7s25f146d107p5
B) [Rn]7s26d107p6
C) [Rn]7s15f146d107p6
D) [Rn]7s27f147d107p5
E) [Rn]7s24f143d107p5
A) [Rn]7s25f146d107p5
B) [Rn]7s26d107p6
C) [Rn]7s15f146d107p6
D) [Rn]7s27f147d107p5
E) [Rn]7s24f143d107p5

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
8
The wavefunction for the 2s orbital in hydrogen is given by 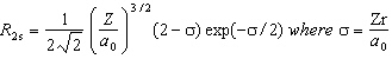 At what radius is there a node?
At what radius is there a node?
A) r = 0
B) r = 2/a0
C) r = a0
D) r = 2a0
E) there are no radial nodes in this function
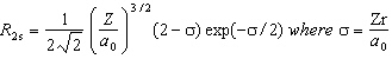 At what radius is there a node?
At what radius is there a node?A) r = 0
B) r = 2/a0
C) r = a0
D) r = 2a0
E) there are no radial nodes in this function

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck
9
Of the following angular components for the wave functions of one-electron atoms, which one(s) must correspond only to s type orbitals?
A)
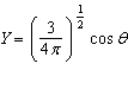
B)
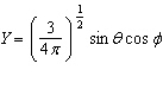
C)
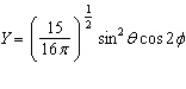
D)
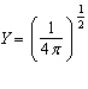
E) B and C
F) None of the above
A)
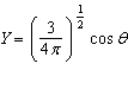
B)
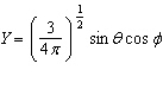
C)
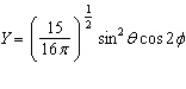
D)
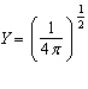
E) B and C
F) None of the above

Unlock Deck
Unlock for access to all 9 flashcards in this deck.
Unlock Deck
k this deck


