Exam 4: Quantum Mechanics, Atomic Structure, Quantum Mechanics and Molecular Structure
Exam 1: The Atom in Modern Chemistry7 Questions
Exam 2: Chemical Formulas, Chemical Equations, and Reaction Yields15 Questions
Exam 3: Introduction to Quantum Mechanics and Chemical Bonding7 Questions
Exam 4: Quantum Mechanics, Atomic Structure, Quantum Mechanics and Molecular Structure9 Questions
Exam 5: Solids, Liquids, Phase Transitions, Gaseous State and Bonding in Organic Molecules10 Questions
Exam 6: Solutions12 Questions
Exam 7: Thermodynamic Processes, Thermochemistry, Spontaneous Processes and Thermodynamic Equilibrium12 Questions
Exam 8: Chemical Equilibrium13 Questions
Exam 9: Acidûbase Equilibria11 Questions
Exam 10: Solubility and Precipitation Equilibria6 Questions
Exam 11: Electrochemistry12 Questions
Exam 12: Chemical Kinetics12 Questions
Exam 13: Inorganic Materials, Structure, Bonding in Solids, Molecular Spectroscopy, Photochemistry and Nuclear Chemistry11 Questions
Select questions type
A MO orbital for a heteronuclear diatomic is given by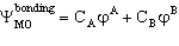 If A is more electronegative than B then you would predict that
If A is more electronegative than B then you would predict that
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
A
Given that Zeff(2s) in Li is 1.26, estimate the energy of the 2s electron in Li in Rhydberg units.
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
A
The wavefunction for the 2s orbital in hydrogen is given by 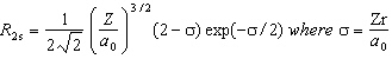 At what radius is there a node?
At what radius is there a node?
Free
(Multiple Choice)
4.8/5  (26)
(26)
Correct Answer:
D
Oxygen is paramagnetic and has a bond order of two. Which of the following represents the ground electronic state for oxygen?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following combinations of atomic orbitals will result in a bonding molecular orbital for a homonuclear diatomic molecule when the z-axis is aligned with the nuclei of the bonded atom?
(Multiple Choice)
4.8/5  (33)
(33)
Of the following angular components for the wave functions of one-electron atoms, which one(s) must correspond only to s type orbitals?
(Multiple Choice)
4.8/5  (30)
(30)
What do you predict of the electron configuration of element 117?
(Multiple Choice)
4.8/5  (43)
(43)
The molecule HeH+ has an antibonding MO of the form
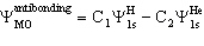 For this you would expect
For this you would expect
(Multiple Choice)
4.9/5  (43)
(43)
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)