Deck 6: Lifes Mainspring: an Introduction to Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/78
Play
Full screen (f)
Deck 6: Lifes Mainspring: an Introduction to Energy
1
What percentage of the energy stored in glucose do humans recover by cellular respiration?
A)100 percent
B)48 percent
C)52 percent
D)37 percent
E)15 percent
A)100 percent
B)48 percent
C)52 percent
D)37 percent
E)15 percent
D
2
During photosynthesis,plants use light energy to synthesize glucose from carbon dioxide.However,plants do not use up energy during photosynthesis; they merely convert it from light energy to chemical energy.This is an illustration of:
A)spontaneous reaction.
B)chemical equilibrium.
C)the second law of thermodynamics.
D)the first law of thermodynamics.
E)increasing entropy.
A)spontaneous reaction.
B)chemical equilibrium.
C)the second law of thermodynamics.
D)the first law of thermodynamics.
E)increasing entropy.
D
3
When we metabolize our food,we produce heat that helps to keep us warm.Which of the following best describes why?
A)Chewing is exothermic; therefore, energy is released in the form of heat when we eat food.
B)When we break down food, all energy in the food is directly released in the form of heat.
C)Producing ATP from ADP is exothermic; therefore, energy is released in the form of heat.
D)When we break down our food, the reactions are not 100 percent efficient; therefore, energy is lost as heat.
A)Chewing is exothermic; therefore, energy is released in the form of heat when we eat food.
B)When we break down food, all energy in the food is directly released in the form of heat.
C)Producing ATP from ADP is exothermic; therefore, energy is released in the form of heat.
D)When we break down our food, the reactions are not 100 percent efficient; therefore, energy is lost as heat.
D
4
Plants provide animals with which of the following?
A)food
B)oxygen
C)carbon dioxide
D)food and oxygen
E)food and carbon dioxide
A)food
B)oxygen
C)carbon dioxide
D)food and oxygen
E)food and carbon dioxide

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following accurately describes energy?
A)something that can cause movement
B)something that produces heat
C)something that has the capacity to do work
D)something that must be eaten
E)something that provides sunlight
A)something that can cause movement
B)something that produces heat
C)something that has the capacity to do work
D)something that must be eaten
E)something that provides sunlight

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
6
Which law of thermodynamics explains the fact that we must eat to gain the energy to perform the functions of life,such as breathing?
A)second law
B)fourth law
C)fifth law
D)first law
E)third law
A)second law
B)fourth law
C)fifth law
D)first law
E)third law

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
7
Kinetic energy is ________ and potential energy is ________.
A)stored energy; energy that is being used
B)stored energy; energy that can't be used
C)energy that can't be used; stored energy
D)energy that is being used; stored energy
A)stored energy; energy that is being used
B)stored energy; energy that can't be used
C)energy that can't be used; stored energy
D)energy that is being used; stored energy

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
8
In the reaction glucose + oxygen → carbon dioxide + heat,the:
A)products have more potential energy than the reactants.
B)products have less potential energy than the reactants.
C)products have the same amount of potential energy as the reactants.
D)entropy has decreased.
A)products have more potential energy than the reactants.
B)products have less potential energy than the reactants.
C)products have the same amount of potential energy as the reactants.
D)entropy has decreased.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is true of energy?
A)It can be stored in chemical bonds.
B)It can be released by forming chemical bonds.
C)It can be transferred from one form to another with 100 percent efficiency.
D)It can be created from nothing.
A)It can be stored in chemical bonds.
B)It can be released by forming chemical bonds.
C)It can be transferred from one form to another with 100 percent efficiency.
D)It can be created from nothing.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
10
You work hard to pedal your bicycle up a steep hill.You rest when you get to the top,then you let your bicycle roll quickly down the other side of the hill.You converted ________ energy into ________ energy,then you converted it back into ________ energy.
A)potential; kinetic; potential
B)kinetic; potential; kinetic
C)heat; kinetic; heat
D)heat; potential; heat
A)potential; kinetic; potential
B)kinetic; potential; kinetic
C)heat; kinetic; heat
D)heat; potential; heat

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
11
Living things are highly ordered.So why does life not violate the second law of thermodynamics?
A)Life can only violate the first law of thermodynamics.
B)Life only needs to follow the first law of thermodynamics.
C)Life takes in energy to maintain order and, in doing so, decreases order elsewhere.
D)Life gives off energy to maintain order and, in doing so, decreases order elsewhere.
A)Life can only violate the first law of thermodynamics.
B)Life only needs to follow the first law of thermodynamics.
C)Life takes in energy to maintain order and, in doing so, decreases order elsewhere.
D)Life gives off energy to maintain order and, in doing so, decreases order elsewhere.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is exergonic?
A)bringing glucose molecules together to form glycogen
B)plants producing glucose from CO₂
C)bringing amino acids together to form proteins
D)cells breaking down glucose into CO₂
A)bringing glucose molecules together to form glycogen
B)plants producing glucose from CO₂
C)bringing amino acids together to form proteins
D)cells breaking down glucose into CO₂

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
13
When you digest the starch in plants into glucose,some energy is lost as heat.This increases the ________ of the universe.
A)energy
B)order
C)entropy
D)potential
E)equilibrium
A)energy
B)order
C)entropy
D)potential
E)equilibrium

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is endergonic?
A)cells breaking down glucose into CO₂
B)the burning of wood
C)the digestion of proteins in the stomach
D)breaking bonds in starch to produce glucose
E)the synthesis of glucose from carbon dioxide and water
A)cells breaking down glucose into CO₂
B)the burning of wood
C)the digestion of proteins in the stomach
D)breaking bonds in starch to produce glucose
E)the synthesis of glucose from carbon dioxide and water

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
15
Energy present in a system that is not usable to do work relates to the system's:
A)thermodynamics.
B)equilibrium.
C)work.
D)entropy.
E)temperature.
A)thermodynamics.
B)equilibrium.
C)work.
D)entropy.
E)temperature.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
16
Glucose can be broken down to provide energy for the beating of cilia.Therefore,the glucose must contain:
A)potential energy.
B)kinetic energy.
C)heat energy.
D)entropy.
A)potential energy.
B)kinetic energy.
C)heat energy.
D)entropy.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following has the most entropy?
A)steam
B)snow
C)an ice cube
D)hot water
E)liquid water
A)steam
B)snow
C)an ice cube
D)hot water
E)liquid water

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
18
The second law of thermodynamics states that:
A)energy is required to bring molecules into a cell against a concentration gradient.
B)energy can be neither created nor destroyed.
C)all living organisms must eat to derive energy.
D)in energy-yielding reactions, matter goes from a more-ordered to a less-ordered state.
A)energy is required to bring molecules into a cell against a concentration gradient.
B)energy can be neither created nor destroyed.
C)all living organisms must eat to derive energy.
D)in energy-yielding reactions, matter goes from a more-ordered to a less-ordered state.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
19
Entropy is the measure of ________ in a system.
A)energy
B)disorder
C)work
D)order
E)heat
A)energy
B)disorder
C)work
D)order
E)heat

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
20
The first law of thermodynamics states that:
A)energy is required to bring molecules into a cell against a concentration gradient.
B)in energy-yielding reactions, matter goes from a more-ordered to a less-ordered state.
C)energy can be neither created nor destroyed.
D)all living organisms must eat to derive energy.
A)energy is required to bring molecules into a cell against a concentration gradient.
B)in energy-yielding reactions, matter goes from a more-ordered to a less-ordered state.
C)energy can be neither created nor destroyed.
D)all living organisms must eat to derive energy.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
21
What makes an enzyme a catalyst?
A)its ability to change shape to fit the substrate
B)it speeds up a chemical reaction but can only be used once
C)it speeds up a chemical reaction but becomes changed by the reaction
D)it speeds up a chemical reaction but is not changed by the reaction
E)its amino acids
A)its ability to change shape to fit the substrate
B)it speeds up a chemical reaction but can only be used once
C)it speeds up a chemical reaction but becomes changed by the reaction
D)it speeds up a chemical reaction but is not changed by the reaction
E)its amino acids

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
22
Coenzymes:
A)serve as catalysts.
B)make proteins.
C)help enzymes to function.
D)break down sugars.
A)serve as catalysts.
B)make proteins.
C)help enzymes to function.
D)break down sugars.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
23
Coupled reactions are:
A)reactions in which endergonic reactions obtain the energy to go forward from exergonic reactions.
B)reactions in which exergonic reactions obtain the energy to go forward from endergonic reactions.
C)any reactions that are accelerated by an enzyme.
D)reactions that lower the activation energy of another reaction.
A)reactions in which endergonic reactions obtain the energy to go forward from exergonic reactions.
B)reactions in which exergonic reactions obtain the energy to go forward from endergonic reactions.
C)any reactions that are accelerated by an enzyme.
D)reactions that lower the activation energy of another reaction.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
24
In the following reaction,which of the following is an example of a substrate? Lactase
Lactose → Glucose + Galactose
A)galactose
B)glucose
C)lactose
D)lactase
Lactose → Glucose + Galactose
A)galactose
B)glucose
C)lactose
D)lactase

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
25
What is the energy currency of cells?
A)ATP
B)glucose
C)enzymes
D)ADP
E)vitamins
A)ATP
B)glucose
C)enzymes
D)ADP
E)vitamins

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
26
The diversity of chemical reactions occurring in a cell depends mostly on certain molecules present in the cells,which are called:
A)enzymes.
B)proteins.
C)cofactors.
D)coenzymes.
E)ribozymes.
A)enzymes.
B)proteins.
C)cofactors.
D)coenzymes.
E)ribozymes.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements concerning enzymes is false?
A)They accelerate the rate of chemical reactions.
B)Many activities in living organisms require multiple enzymes.
C)One enzyme will work on many different substrate molecules to produce many different products.
D)They can carry out hundreds of chemical transformations per second.
A)They accelerate the rate of chemical reactions.
B)Many activities in living organisms require multiple enzymes.
C)One enzyme will work on many different substrate molecules to produce many different products.
D)They can carry out hundreds of chemical transformations per second.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
28
The energy released during ________ reactions can be used to drive ________ reactions,and this is called ________ reactions.
A)endergonic; exergonic; coupled
B)exergonic; endergonic; coupled
C)endergonic; exergonic; mutually dependent
D)exergonic; endergonic; mutually dependent
A)endergonic; exergonic; coupled
B)exergonic; endergonic; coupled
C)endergonic; exergonic; mutually dependent
D)exergonic; endergonic; mutually dependent

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
29
Enzymes work to speed up a chemical reaction by:
A)supplying the energy needed for the reaction.
B)increasing the activation energy of the reaction.
C)lowering the activation energy of the reaction.
D)attaching coenzymes to the substrate.
E)heating up the substrate.
A)supplying the energy needed for the reaction.
B)increasing the activation energy of the reaction.
C)lowering the activation energy of the reaction.
D)attaching coenzymes to the substrate.
E)heating up the substrate.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
30
Which would be an example of a catalyst in action?
A)a glycoprotein binding to a cell
B)a protein converting glucose and fructose into sucrose without being changed itself
C)glucose and galactose binding to form lactose
D)a phosphate group attached to a protein changing its shape
A)a glycoprotein binding to a cell
B)a protein converting glucose and fructose into sucrose without being changed itself
C)glucose and galactose binding to form lactose
D)a phosphate group attached to a protein changing its shape

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements concerning enzymes is false?
A)They function as catalysts.
B)The active site of an enzyme has a shape that is specific for its given substrate.
C)They increase the activation energy required for chemical reactions to proceed.
D)Many enzymes utilize coenzymes, such as vitamins.
A)They function as catalysts.
B)The active site of an enzyme has a shape that is specific for its given substrate.
C)They increase the activation energy required for chemical reactions to proceed.
D)Many enzymes utilize coenzymes, such as vitamins.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
32
Most enzymes are:
A)proteins.
B)nucleic acids.
C)lipids.
D)vitamins.
E)carbohydrates.
A)proteins.
B)nucleic acids.
C)lipids.
D)vitamins.
E)carbohydrates.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
33
The structure of ATP includes each of the following except:
A)the nitrogenous base adenosine.
B)the sugar ribose.
C)the nitrogenous base adenine.
D)phosphate groups.
A)the nitrogenous base adenosine.
B)the sugar ribose.
C)the nitrogenous base adenine.
D)phosphate groups.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
34
The substance that is worked on by an enzyme is called:
A)product.
B)coenzyme.
C)inhibitor.
D)substrate.
A)product.
B)coenzyme.
C)inhibitor.
D)substrate.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
35
You have a friend who tells you she is lactose intolerant.She asks you to explain what this means.You say to her:
A)"You cannot digest milk because you cannot absorb it in your intestines."
B)"You are allergic to milk."
C)"You cannot digest milk because you do not have the enzyme to break down the sugar in the milk."
D)"You cannot digest the milk because you do not have the enzyme to break down the protein in the milk."
E)"You cannot digest the milk because you do not have the enzyme to break down the lipid in the milk."
A)"You cannot digest milk because you cannot absorb it in your intestines."
B)"You are allergic to milk."
C)"You cannot digest milk because you do not have the enzyme to break down the sugar in the milk."
D)"You cannot digest the milk because you do not have the enzyme to break down the protein in the milk."
E)"You cannot digest the milk because you do not have the enzyme to break down the lipid in the milk."

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
36
In a ________,multiple enzymes are working together in a multistep process.
A)feedback loop
B)coupled reaction
C)allosteric pathway
D)metabolic pathway
A)feedback loop
B)coupled reaction
C)allosteric pathway
D)metabolic pathway

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
37
Metabolism is the sum of all the:
A)ATP a cell contains.
B)energy a cell uses.
C)chemical reactions that a cell carries out.
D)vitamins a cell contains.
E)enzymes a cell contains.
A)ATP a cell contains.
B)energy a cell uses.
C)chemical reactions that a cell carries out.
D)vitamins a cell contains.
E)enzymes a cell contains.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
38
Gasoline will not burn in your car's engine unless it is ignited with a spark from a sparkplug.In this situation,the spark is providing:
A)activation energy.
B)hydrolytic energy.
C)coenzyme energy.
D)enzymatic energy.
E)entropy.
A)activation energy.
B)hydrolytic energy.
C)coenzyme energy.
D)enzymatic energy.
E)entropy.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
39
Some enzymes contain molecules in the active site that help facilitate chemical transformations.These molecules are called:
A)coenzymes.
B)products.
C)substrates.
D)co-substrates.
A)coenzymes.
B)products.
C)substrates.
D)co-substrates.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
40
In the ATP/ADP cycle:
A)food provides the energy needed to link phosphate to ADP to make ATP.
B)food provides the energy needed to break down ATP into ADP.
C)converting ADP into ATP is an energy-yielding reaction, whereas converting ATP into ADP is an energy-requiring reaction.
D)after powering a reaction, ADP becomes ATP.
A)food provides the energy needed to link phosphate to ADP to make ATP.
B)food provides the energy needed to break down ATP into ADP.
C)converting ADP into ATP is an energy-yielding reaction, whereas converting ATP into ADP is an energy-requiring reaction.
D)after powering a reaction, ADP becomes ATP.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
41
Cells can harvest the energy released during exergonic reactions to drive endergonic reactions.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
42
People with high cholesterol levels often take drugs in an attempt to lower their cholesterol levels.One such drug is Lipitor.How does this drug work?
A)It enhances the activity of enzymes that help break down cholesterol in the body.
B)It prevents cholesterol from the diet from being absorbed in the small intestine.
C)It acts as a competitive inhibitor by binding to the active site of enzymes that normally produce cholesterol.
D)It increases the activation energy of enzymes needed to produce cholesterol.
A)It enhances the activity of enzymes that help break down cholesterol in the body.
B)It prevents cholesterol from the diet from being absorbed in the small intestine.
C)It acts as a competitive inhibitor by binding to the active site of enzymes that normally produce cholesterol.
D)It increases the activation energy of enzymes needed to produce cholesterol.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
43
In allosteric enzyme regulation,which of the following is false about the molecules that can inhibit enzyme activity?
A)They can cause the active site to have a poor shape for binding substrate.
B)They can increase substrate binding.
C)They cause a change in the enzyme's shape.
D)They can bind to a site other than the active site.
A)They can cause the active site to have a poor shape for binding substrate.
B)They can increase substrate binding.
C)They cause a change in the enzyme's shape.
D)They can bind to a site other than the active site.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
44
Endergonic reactions release energy.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
45
Allosteric regulation depends on inhibitors binding to the active site of enzymes.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
46
What mechanism is used to finely tune enzyme activity according to the needs of the cell?
A)competitive inhibition
B)coupled reactions
C)enzyme specificity
D)allosteric regulation
A)competitive inhibition
B)coupled reactions
C)enzyme specificity
D)allosteric regulation

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is true of allosteric enzymes?
A)Their activity can be reduced when the product binds the enzyme.
B)Their activity can be reduced when the substrate binds the enzyme.
C)Their coenzyme is removed when the product binds the enzyme.
D)Their activity can be increased by competitive inhibitors.
A)Their activity can be reduced when the product binds the enzyme.
B)Their activity can be reduced when the substrate binds the enzyme.
C)Their coenzyme is removed when the product binds the enzyme.
D)Their activity can be increased by competitive inhibitors.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following describes a transition state molecule?
A)the distorted shape the product takes while being released from the enzyme
B)the distorted shape the substrate takes when being converted by an enzyme into product
C)the distorted shape the enzyme takes when converting substrate into product
D)the distorted shape a coenzyme takes while it is bound to an enzyme
A)the distorted shape the product takes while being released from the enzyme
B)the distorted shape the substrate takes when being converted by an enzyme into product
C)the distorted shape the enzyme takes when converting substrate into product
D)the distorted shape a coenzyme takes while it is bound to an enzyme

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
49
The function of chymotrypsin is to:
A)break down proteins into amino acids.
B)synthesize glycogen.
C)synthesize proteins.
D)break down glycogen into glucose.
A)break down proteins into amino acids.
B)synthesize glycogen.
C)synthesize proteins.
D)break down glycogen into glucose.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
50
Methyl alcohol,also known as wood alcohol,is a common solvent and paint remover.It is poisonous if accidentally swallowed.The enzyme alcohol dehydrogenase in the liver converts methyl alcohol into formaldehyde,which then gets converted into a toxic product.Grain alcohol,ethyl alcohol,is also acted upon by alcohol dehydrogenase.One antidote for methyl alcohol poisoning is to make a person drink a lot of ethyl alcohol.This blocks the active site of the enzyme so that it can't bind to and break down the methyl alcohol.In this capacity,the ethyl alcohol is acting as a/an:
A)allosteric regulator.
B)coenzyme.
C)competitive inhibtor.
D)precursor.
A)allosteric regulator.
B)coenzyme.
C)competitive inhibtor.
D)precursor.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
51
Enzymes themselves are altered in the process of catalyzing chemical transformations.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
52
Given enough time,sucrose can spontaneously break down into fructose and glucose in a glass of water.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is true of vitamins?
A)They serve as a major energy source for cells.
B)They are the main component of enzymes.
C)They can serve as enzymes.
D)They can serve as coenzymes.
A)They serve as a major energy source for cells.
B)They are the main component of enzymes.
C)They can serve as enzymes.
D)They can serve as coenzymes.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
54
It is possible for a cell to harvest 100 percent of the energy from a chemical reaction to produce movement.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
55
When a product binds to an allosteric enzyme to slow its reaction,it does which of the following?
A)binds to the active site, blocking the binding of substrate
B)binds to a site other than the active site, changing the shape of the active site and decreasing the binding of substrate
C)binds to the substrate, blocking its binding of the active site
D)binds to the product production site, stopping the production of product
A)binds to the active site, blocking the binding of substrate
B)binds to a site other than the active site, changing the shape of the active site and decreasing the binding of substrate
C)binds to the substrate, blocking its binding of the active site
D)binds to the product production site, stopping the production of product

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
56
Enzymes increase the activation energy needed for a particular reaction.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
57
Where do substrates bind to enzymes?
A)in the active site
B)anywhere
C)in the substrate groove
D)in the effector site
A)in the active site
B)anywhere
C)in the substrate groove
D)in the effector site

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
58
Why is allosteric regulation important to a cell?
A)It allows the cell to control how much of a metabolic product is produced.
B)It allows the cell to switch to other sources of energy besides ATP.
C)It reduces the cell's need for coenzymes.
D)It allows the cell to control how much activation energy is required for enzymatic reactions.
A)It allows the cell to control how much of a metabolic product is produced.
B)It allows the cell to switch to other sources of energy besides ATP.
C)It reduces the cell's need for coenzymes.
D)It allows the cell to control how much activation energy is required for enzymatic reactions.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
59
Only eukaryotic cells depend on ATP.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
60
Refer to the figure below, and then answer the question that follows.
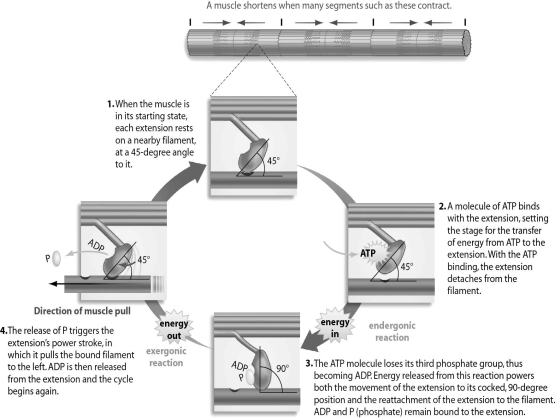
You have a job as an assistant in a morgue.You are startled (almost to death)when a corpse that recently came in suddenly sits up and remains very stiff.This muscle contraction and stiffness in a dead body is known as rigor mortis.Using your knowledge of the ADP/ATP cycle and its role in muscle contraction,explain why rigor mortis occurs.

You have a job as an assistant in a morgue.You are startled (almost to death)when a corpse that recently came in suddenly sits up and remains very stiff.This muscle contraction and stiffness in a dead body is known as rigor mortis.Using your knowledge of the ADP/ATP cycle and its role in muscle contraction,explain why rigor mortis occurs.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
61
Phosphofructokinase is an enzyme involved in glycolysis,which is a metabolic pathway involved in ATP production.It can be allosterically regulated by both ATP and ADP.Why is regulating the activity of this enzyme important? Predict what will happen to the rate of reaction when ATP levels in the cell are high,and predict what will happen when the levels of ADP in the cell are high.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
62
The regulation of an enzyme's activity by means of a molecule binding to a site on the enzyme other than its active site is called ________.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
63
Explain how vitamins play a role in cellular metabolism.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
64
A ripped piece of paper will never spontaneously repair itself.Explain the law of thermodynamics that explains why this is so.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
65
The study of the thermodynamics of biology is known as ________.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
66
It has been hypothesized that,if the sun disappeared,all life would end.Other than the obvious drop in temperature,why would humans probably die if the sun suddenly stopped shining?

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
67
Molecules that facilitate the work of enzymes by binding with them are ________.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
68
Explain why enzymes are involved in exergonic reactions,such as lactose breaking down into glucose and galactose,and whether these reactions can occur spontaneously.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
69
Explain why it takes energy to create starch from glucose,but it does not take energy to create glucose from starch.In your explanation,describe the law of thermodynamics that explains this,and describe how.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
70
Individuals with the genetic disease phenylketonuria (PKU)lack a single enzyme called phenylalanine hydroxylase,which converts the amino acid phenylalanine to the amino acid tyrosine.If this conversion does not happen,phenylalanine builds up and becomes toxic to the central nervous system,causing serious problems for the individual.The management of this disease is to avoid phenylalanine by severely limiting the amount of protein in the diet.How would this help?

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
71
A reaction that requires the input of energy to move forward is ________.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
72
Would ATP be the energy currency of the cell if the phosphate groups the cell contains were not charged? Why or why not?

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
73
Refer to the figure below, and then answer the question that follows.
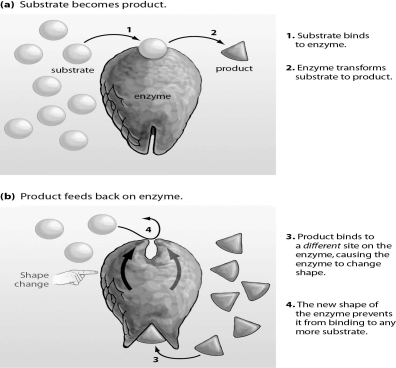
The figure above represents allosteric inhibition.Based on the figure,what could be done to allow the enzyme to function again?
A)add more enzyme
B)add more substrate
C)remove some of the product
D)remove some of the substrate

The figure above represents allosteric inhibition.Based on the figure,what could be done to allow the enzyme to function again?
A)add more enzyme
B)add more substrate
C)remove some of the product
D)remove some of the substrate

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
74
You have been asked to explain to a high school class the second law of thermodynamics and how this law affects the students' lives.Using your own words,describe the second law of thermodynamics,and give an example (other than the examples described in the chapter).

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
75
Refer to the figure below, and then answer the question that follows.
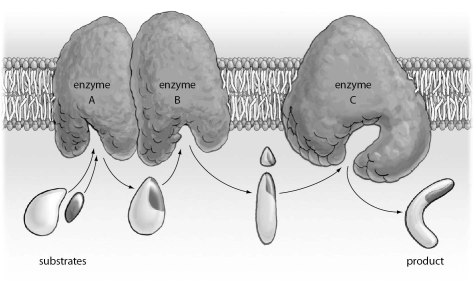
The figure above represents:
A)a metabolic pathway.
B)lowering activation energy.
C)competitive inhibition.
D)allosteric regulation.

The figure above represents:
A)a metabolic pathway.
B)lowering activation energy.
C)competitive inhibition.
D)allosteric regulation.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
76
You have been asked to explain to a high school class the first law of thermodynamics and how this law affects the students' lives.Using your own words,describe the first law of thermodynamics,and give an example (other than the examples described in the chapter).

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
77
A ________ is a set of enzymatically controlled steps that results in the completion of a product or process in an organism.

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck
78
Match between columns

Unlock Deck
Unlock for access to all 78 flashcards in this deck.
Unlock Deck
k this deck


