Deck 12: Acids Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 12: Acids Bases
1
An aqueous solution is found to have an H+ concentration of 2.0 × 10-4 M.What is the concentration of OH- in this solution?
A) 5.0 × 10-11 M
B) 2.0 × 10-18 M
C) 2.0 × 1010 M
D) 4.0 M
A) 5.0 × 10-11 M
B) 2.0 × 10-18 M
C) 2.0 × 1010 M
D) 4.0 M
5.0 × 10-11 M
2
In the following equilibrium,which species is the CONJUGATE BASE of compound b? 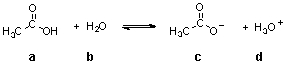
A) a
B) b
C) c
D) d

A) a
B) b
C) c
D) d
d
3
A compound that increases the concentration of hydroxide ion in an aqueous solution is a(n):
A) acid.
B) base.
C) salt.
D) solvent.
A) acid.
B) base.
C) salt.
D) solvent.
base.
4
The pH of an unknown solution is 8.5.Its [OH-] is:
A) 3.2 × 10-6 M.
B) 3.2 × 10-9 M.
C) 3.2 × 105 M.
D) 3.2 M.
A) 3.2 × 10-6 M.
B) 3.2 × 10-9 M.
C) 3.2 × 105 M.
D) 3.2 M.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
What is the pH of a solution if [H+] = 1.0 × 10-9 M?
A) 6.0
B) 9.0
C) 8.6
D) 5.4
A) 6.0
B) 9.0
C) 8.6
D) 5.4

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
In the following equilibrium,which species is the CONJUGATE BASE of compound a? 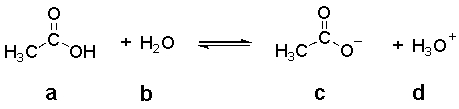
A) a
B) b
C) c
D) d
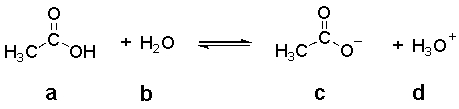
A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
The net ionic equation for the reaction Zn (s)+ 2 HNO3 (aq) H2(g)+ Zn(NO3)2 (aq)is:
A) Zn (s)+ H+ (aq) H2(g)+ Zn2+ (aq).
B) Zn (s)+ 2 H+ (aq) H2(g)+ Zn2+ (aq).
C) Zn (s)+ 2 H+ (aq) H2(g)+ Zn+ (aq).
D) Zn (s)+ H+ (aq) H2(g)+ Zn+ (aq).
A) Zn (s)+ H+ (aq) H2(g)+ Zn2+ (aq).
B) Zn (s)+ 2 H+ (aq) H2(g)+ Zn2+ (aq).
C) Zn (s)+ 2 H+ (aq) H2(g)+ Zn+ (aq).
D) Zn (s)+ H+ (aq) H2(g)+ Zn+ (aq).

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
Predict the products for HCl (aq)+ NaOH (aq)
A) NaCl (aq)+ H2O (l)
B) NaCl (aq)+ NaOH (aq)
C) NaCl (aq)+ HCl (aq)
D) NaCl (aq)+ H2 (g)
A) NaCl (aq)+ H2O (l)
B) NaCl (aq)+ NaOH (aq)
C) NaCl (aq)+ HCl (aq)
D) NaCl (aq)+ H2 (g)

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
An aqueous solution is found to have an H+ concentration of 7.8 × 10-2 M.What is the concentration of OH- in this solution?
A) 1.3 × 10-13 M
B) 7.8 × 1012 M
C) 2.0 × 1010 M
D) 7.8 M
A) 1.3 × 10-13 M
B) 7.8 × 1012 M
C) 2.0 × 1010 M
D) 7.8 M

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
Which acid can undergo up to a second ionization?
A) H2SO4 (aq)
B) HNO3 (aq)
C) HNO2 (aq)
D) HCl (aq)
A) H2SO4 (aq)
B) HNO3 (aq)
C) HNO2 (aq)
D) HCl (aq)

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the Brønsted base in this reaction. H2O (l)+ HNO3 (aq) H3O+ (aq)+ NO3- (aq)
A) H2O (l)
B) HNO3 (aq)
C) H3O+ (aq)
D) NO3- (aq)
A) H2O (l)
B) HNO3 (aq)
C) H3O+ (aq)
D) NO3- (aq)

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
Acids that completely ionize in water are:
A) strong bases.
B) weak bases.
C) strong acids.
D) weak acids.
A) strong bases.
B) weak bases.
C) strong acids.
D) weak acids.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
The neutralization reaction of barium hydroxide with aqueous hydrochloric acid (HCl)leads to what two products?
A) H2O and BaCl
B) Ba(ClO2)2 and H2
C) HCl and BaOCl
D) BaCl2 and H2O
A) H2O and BaCl
B) Ba(ClO2)2 and H2
C) HCl and BaOCl
D) BaCl2 and H2O

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the Brønsted acid in this reaction. H2SO4 (aq)+ HNO3 (aq) HSO4- (aq)+ H2NO3+ (aq)
A) H2SO4 (aq)
B) HNO3 (aq)
C) HSO4- (aq)
D) H2NO3+ (aq)
A) H2SO4 (aq)
B) HNO3 (aq)
C) HSO4- (aq)
D) H2NO3+ (aq)

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
Acids that partially ionize in water are:
A) strong bases.
B) weak bases.
C) strong acids.
D) weak acids.
A) strong bases.
B) weak bases.
C) strong acids.
D) weak acids.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
The pH of an unknown solution is 2.1.Its [OH-] is:
A) 0.0079 M.
B) 1.3 × 10-12 M.
C) 3.2 × 102 M.
D) 3.2 M.
A) 0.0079 M.
B) 1.3 × 10-12 M.
C) 3.2 × 102 M.
D) 3.2 M.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
Acid and base combine to form:
A) salt and water.
B) gas and water.
C) salt and gas.
D) only ionic compounds.
A) salt and water.
B) gas and water.
C) salt and gas.
D) only ionic compounds.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
Which acid will fully ionize in water?
A) HClO
B) HF
C) HCl
D) HC2Cl3O2
A) HClO
B) HF
C) HCl
D) HC2Cl3O2

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
A compound that is a proton (H+)donor is a(n):
A) salt.
B) solvent.
C) base.
D) acid.
A) salt.
B) solvent.
C) base.
D) acid.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
The net ionic equation for the reaction Cu (s)+ 2 HNO3 (aq) H2(g)+ Cu(NO3)2 (aq)is:
A) Cu (s)+ H+ (aq) H2(g)+ Cu2+ (aq).
B) Cu (s)+ 2 H+ (aq) H2(g)+ Cu2+ (aq).
C) Cu (s)+ 2 H+ (aq) H2(g)+ Cu+ (aq).
D) Cu (s)+ H+ (aq) H2(g)+ Cu+ (aq).
A) Cu (s)+ H+ (aq) H2(g)+ Cu2+ (aq).
B) Cu (s)+ 2 H+ (aq) H2(g)+ Cu2+ (aq).
C) Cu (s)+ 2 H+ (aq) H2(g)+ Cu+ (aq).
D) Cu (s)+ H+ (aq) H2(g)+ Cu+ (aq).

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
Which statement is FALSE?
A) Buffer systems keep pH within a narrow range.
B) A buffer is a solution containing a mixture of acidic and basic components that resist changes in pH.
C) An appropriate buffer is a mixture of a strong acid and its conjugate base.
D) An appropriate buffer is a mixture of a weak acid and its conjugate base.
A) Buffer systems keep pH within a narrow range.
B) A buffer is a solution containing a mixture of acidic and basic components that resist changes in pH.
C) An appropriate buffer is a mixture of a strong acid and its conjugate base.
D) An appropriate buffer is a mixture of a weak acid and its conjugate base.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
Which combination would make a buffer solution?
A) 0.1 M HCl and 0.1 M NaCl
B) 0.1 M H2CO3 and 0.1 M NaHCO3
C) 0.4 M HNO3 and 0.4 M NaNO3
D) 0.15 M H2SO4 and 0.4 M CaSO4
A) 0.1 M HCl and 0.1 M NaCl
B) 0.1 M H2CO3 and 0.1 M NaHCO3
C) 0.4 M HNO3 and 0.4 M NaNO3
D) 0.15 M H2SO4 and 0.4 M CaSO4

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
A 50.0-mL sample of HNO3 is neutralized by reaction with 120.0 mL of 2.50 M NaOH.What is the concentration of HNO3 in the unknown sample?
A) 6.00 M
B) 1.04 M
C) 0.960 M
D) 1.55 M
A) 6.00 M
B) 1.04 M
C) 0.960 M
D) 1.55 M

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
A buffer solution contains a mixture of carbonic acid (H2CO3)and potassium hydrogen carbonate (KHCO3).What reaction will take place if H+ is added to this solution?
A) H2CO3 (aq)+ H+ (aq) H3CO3+ (aq)
B) HCO3- (aq)+ H+ (aq) H2CO3 (aq)
C) KHCO3 (aq)+ H+ (aq) KH2CO3 (aq)
D) CO32- (aq)+ H+ (aq) HC2H3O2 (aq)
A) H2CO3 (aq)+ H+ (aq) H3CO3+ (aq)
B) HCO3- (aq)+ H+ (aq) H2CO3 (aq)
C) KHCO3 (aq)+ H+ (aq) KH2CO3 (aq)
D) CO32- (aq)+ H+ (aq) HC2H3O2 (aq)

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
A 25.0-mL sample of HBr is neutralized by reaction with 20.0 mL of 3.00 M KOH.What is the concentration of HBr in the unknown sample?
A) 3.8 M
B) 2.4 M
C) 1.7 M
D) 0.55 M
A) 3.8 M
B) 2.4 M
C) 1.7 M
D) 0.55 M

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
A buffer solution contains a mixture of acetic acid (HC2H3O2)and potassium acetate (KC2H3O2).What reaction will take place if H+ is added to this solution?
A) HC2H3O2 (aq)+ H+ (aq) H2C2H3O2+ (aq)
B) C2H3O2- (aq)+ H+ (aq) H2C2H3O2+ (aq)
C) KC2H3O2 (aq)+ H+ (aq) KHC2H3O2 (aq)
D) C2H3O2- (aq)+ H+ (aq) HC2H3O2 (aq)
A) HC2H3O2 (aq)+ H+ (aq) H2C2H3O2+ (aq)
B) C2H3O2- (aq)+ H+ (aq) H2C2H3O2+ (aq)
C) KC2H3O2 (aq)+ H+ (aq) KHC2H3O2 (aq)
D) C2H3O2- (aq)+ H+ (aq) HC2H3O2 (aq)

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
Which solution will turn litmus paper blue?
A) 0.5 M NaOH
B) 2.1 M HCl
C) 4.5 M HC2H3O2
D) 0.125 M HBr
A) 0.5 M NaOH
B) 2.1 M HCl
C) 4.5 M HC2H3O2
D) 0.125 M HBr

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
Which combination would make the MOST effective buffer solution?
A) 0.1 M H2CO3 and 1.0 M NaHCO3
B) 0.1 M H2CO3 and 0.1 M NaHCO3
C) 0.1 M H2CO3 and 2.0 M NaHCO3
D) 0.15 M H2CO3 and 0.015 M NaHCO3
A) 0.1 M H2CO3 and 1.0 M NaHCO3
B) 0.1 M H2CO3 and 0.1 M NaHCO3
C) 0.1 M H2CO3 and 2.0 M NaHCO3
D) 0.15 M H2CO3 and 0.015 M NaHCO3

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
A 50.0-mL sample of H2SO4 is neutralized with NaOH solution having a concentration of 2.00 M.If 35.5 mL of NaOH are required to complete the neutralization,what is the concentration of the sulfuric acid solution?
A) 0.710 M
B) 2.84 M
C) 1.41 M
D) 0.355 M
A) 0.710 M
B) 2.84 M
C) 1.41 M
D) 0.355 M

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
Which solution will turn litmus paper red?
A) 0.5 M KOH
B) 2.1 M Mg(OH)2
C) 4.5 M HC2H3O2
D) 0.125 M KNO3
A) 0.5 M KOH
B) 2.1 M Mg(OH)2
C) 4.5 M HC2H3O2
D) 0.125 M KNO3

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


