Exam 12: Acids Bases
Exam 1: Foundations30 Questions
Exam 2: Measurement26 Questions
Exam 3: Atoms30 Questions
Exam 4: Light and Electronic Structure30 Questions
Exam 5: Chemical Bonds Compounds56 Questions
Exam 6: Chemical Reactions47 Questions
Exam 7: Mass Stoichiometry44 Questions
Exam 8: Energy39 Questions
Exam 9: Covalent Bonding and Molecules30 Questions
Exam 10: Solids, Liquids, and Gases30 Questions
Exam 11: Solutions30 Questions
Exam 12: Acids Bases30 Questions
Exam 13: Reaction Rates Equilibrium40 Questions
Exam 14: Oxidation-Reduction Reactions52 Questions
Exam 15: Organic Chemistry and Biomolecules65 Questions
Exam 16: Nuclear Chemistry52 Questions
Select questions type
A buffer solution contains a mixture of acetic acid (HC2H3O2)and potassium acetate (KC2H3O2).What reaction will take place if H+ is added to this solution?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
D
The pH of an unknown solution is 2.1.Its [OH-] is:
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
B
A compound that is a proton (H+)donor is a(n):
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
D
In the following equilibrium,which species is the CONJUGATE BASE of compound b? 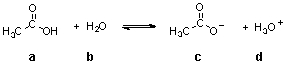
(Multiple Choice)
4.9/5  (32)
(32)
An aqueous solution is found to have an H+ concentration of 2.0 × 10-4 M.What is the concentration of OH- in this solution?
(Multiple Choice)
4.7/5  (39)
(39)
A 25.0-mL sample of HBr is neutralized by reaction with 20.0 mL of 3.00 M KOH.What is the concentration of HBr in the unknown sample?
(Multiple Choice)
4.8/5  (34)
(34)
Which combination would make the MOST effective buffer solution?
(Multiple Choice)
4.8/5  (34)
(34)
The neutralization reaction of barium hydroxide with aqueous hydrochloric acid (HCl)leads to what two products?
(Multiple Choice)
4.8/5  (36)
(36)
In the following equilibrium,which species is the CONJUGATE BASE of compound a? 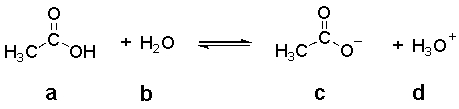
(Multiple Choice)
4.9/5  (36)
(36)
The net ionic equation for the reaction Cu (s)+ 2 HNO3 (aq) H2(g)+ Cu(NO3)2 (aq)is:
(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 30
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)