Deck 19: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/127
Play
Full screen (f)
Deck 19: Electrochemistry
1
Choose the INCORRECT statement.
A)An electrode is often a strip of metal.
B)An electrode in a solution of its ions is a half cell.
C)An electrochemical cell is a half cell.
D)The electromotive force (emf)is the cell potential.
E)The cell potential is the potential difference between the half cells.
A)An electrode is often a strip of metal.
B)An electrode in a solution of its ions is a half cell.
C)An electrochemical cell is a half cell.
D)The electromotive force (emf)is the cell potential.
E)The cell potential is the potential difference between the half cells.
An electrochemical cell is a half cell.
2
In a zinc-lead cell the reaction is:
Pb2+(aq)+ Zn(s)→ Pb(s)+ Zn2+(aq)E° = 0.637 V
Which of the following statements about this cell is FALSE?
A)The zinc electrode is the cathode.
B)The reaction will go in the direction indicated.
C)The shorthand notation is Zn(s)∣ Zn2+(aq) Pb2+(aq)∣ Pb(s).
Pb2+(aq)∣ Pb(s).
D)The actual cell voltage is less than +0.637 volts because of concentration polarization and possible other factors.
E)The lead electrode is positively charged.
Pb2+(aq)+ Zn(s)→ Pb(s)+ Zn2+(aq)E° = 0.637 V
Which of the following statements about this cell is FALSE?
A)The zinc electrode is the cathode.
B)The reaction will go in the direction indicated.
C)The shorthand notation is Zn(s)∣ Zn2+(aq)
 Pb2+(aq)∣ Pb(s).
Pb2+(aq)∣ Pb(s).D)The actual cell voltage is less than +0.637 volts because of concentration polarization and possible other factors.
E)The lead electrode is positively charged.
The zinc electrode is the cathode.
3
The active metal is called a sacrificial cathode.
False
4
Choose the INCORRECT statement.
A)SHE stands for Standard Helium Electrodes.
B)E° is the standard electrode potential.
C)The standard electrode potential is the reduction potential of a half cell.
D)The standard cell potential is the potential difference between two half cells.
E)E°cell is the standard cell potential.
A)SHE stands for Standard Helium Electrodes.
B)E° is the standard electrode potential.
C)The standard electrode potential is the reduction potential of a half cell.
D)The standard cell potential is the potential difference between two half cells.
E)E°cell is the standard cell potential.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
5
Choose the correct statement based on the following oxidation potentials.
Mg/Mg2+ +2.37 v Fe/Fe2+ +0.44 v Cu/Cu2+ -0.34 v
Zn/Zn2+ +0.76 v Sn/Sn2+ +0.14 v Ag/Ag+ -0.80 v
A)Mg will not displace Zn2+ from solution
B)Cu will displace Sn2+ from solution
C)Fe will displace Zn2+ from solution
D)Fe will displace H+ from solution
E)Sn will displace Fe2+ from solution
Mg/Mg2+ +2.37 v Fe/Fe2+ +0.44 v Cu/Cu2+ -0.34 v
Zn/Zn2+ +0.76 v Sn/Sn2+ +0.14 v Ag/Ag+ -0.80 v
A)Mg will not displace Zn2+ from solution
B)Cu will displace Sn2+ from solution
C)Fe will displace Zn2+ from solution
D)Fe will displace H+ from solution
E)Sn will displace Fe2+ from solution

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
6
Choose the INCORRECT statement.
A)Electrons are transferred in oxidation-reduction reactions.
B)A strip of metal in a solution of ions of that metal is an electrode potential.
C)Reduction is the gain of electrons.
D)Oxidation is the loss if electrons.
E)A salt bridge maintains electrical contact between two half cells.
A)Electrons are transferred in oxidation-reduction reactions.
B)A strip of metal in a solution of ions of that metal is an electrode potential.
C)Reduction is the gain of electrons.
D)Oxidation is the loss if electrons.
E)A salt bridge maintains electrical contact between two half cells.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
7
In a galvanic cell,oxidation occurs at the cathode.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
8
In a galvanic cell,oxidation occurs at the:
A)anode
B)cathode
C)salt bridge
D)electrolyte
E)in cathodic space
A)anode
B)cathode
C)salt bridge
D)electrolyte
E)in cathodic space

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
9
Corrosion of metals is an oxidation-reduction process.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
10
Choose the INCORRECT statement.
A)A cell diagram is a symbolic way to show cell components.
B)An anode is where oxidation occurs.
C)A cathode is where reduction occurs.
D)Half cells in a cell diagram are separated by a single vertical line.
E)The anode is on the left in a cell diagram.
A)A cell diagram is a symbolic way to show cell components.
B)An anode is where oxidation occurs.
C)A cathode is where reduction occurs.
D)Half cells in a cell diagram are separated by a single vertical line.
E)The anode is on the left in a cell diagram.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
11
Choose the INCORRECT statement.
A)A double vertical line separates half cells in a cell diagram.
B)A single vertical line separates phases in a cell diagram.
C)A voltaic cell is a galvanic cell.
D)Voltaic cells produce an electron flow.
E)Electrolytic cells are cells where electron flow is caused by spontaneous reactions.
A)A double vertical line separates half cells in a cell diagram.
B)A single vertical line separates phases in a cell diagram.
C)A voltaic cell is a galvanic cell.
D)Voltaic cells produce an electron flow.
E)Electrolytic cells are cells where electron flow is caused by spontaneous reactions.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
12
The Ecell for the following concentration cell at 25 °C is 0.006 V.
Pt ∣ H2 (g,0.025 atm)∣ H+ (0.012 M)
H+ (1 M)∣ H2 (g,1 atm)∣ Pt
2 H+(aq)+ 2 e- → H2(g)E° = 0.V
Pt ∣ H2 (g,0.025 atm)∣ H+ (0.012 M)

H+ (1 M)∣ H2 (g,1 atm)∣ Pt
2 H+(aq)+ 2 e- → H2(g)E° = 0.V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
13
Cathodic protection is attaching a more active metal to the protected metal.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
14
A primary battery is recharged by passing electricity through the battery.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
15
E°cell is the standard cell potential.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
16
In the electrolysis of water,oxygen is evolved at the cathode.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
17
When Ecell < 0,the reaction is spontaneous.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
18
Choose the INCORRECT statement.
A)When a half reaction is reversed,the sign of the potential is changed.
B)Reversing a half reaction makes it a reduction potential.
C)Each electrochemical cell consists of a reduction half cell and an oxidation half cell.
D)A voltaic cell is also called a battery.
E)The potential difference of a cell is the voltage of the cell.
A)When a half reaction is reversed,the sign of the potential is changed.
B)Reversing a half reaction makes it a reduction potential.
C)Each electrochemical cell consists of a reduction half cell and an oxidation half cell.
D)A voltaic cell is also called a battery.
E)The potential difference of a cell is the voltage of the cell.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
19
The hydrogen standard electrode is the most convenient standard electrode to use.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
20
Primary batteries' cell reactions cannot be reversed.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
21
For the reaction: Mg(s)+ AgNO3(aq)→ Ag(s)+ Mg(NO3)2(aq)
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Estimate Keq at 25 °C.
A)5.7 × 10106
B)2.0 × 1053
C)3.7 × 1052
D)1.1 × 1027
E)10-107
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Estimate Keq at 25 °C.
A)5.7 × 10106
B)2.0 × 1053
C)3.7 × 1052
D)1.1 × 1027
E)10-107

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following are commonly used as alternate standard electrodes:
I.Ag(s)| AgCl(s)| Cl- ((aq,1.0 M)
II.Hg(l)| HgCl2(s)| Cl- (aq,1.0 M)
III.Ag(s)| AgCl(s)| Cl- ((aq,1.0 M)
IV.Hg(l)| Hg2Cl2(s)| Cl- (aq,1.0 M)
V.Hg(s)| Hg2Cl2(s)| Cl- (aq,1.0 M)
A)I and V
B)II and IV
C)I and IV
D)II and III
E)I and III
I.Ag(s)| AgCl(s)| Cl- ((aq,1.0 M)
II.Hg(l)| HgCl2(s)| Cl- (aq,1.0 M)
III.Ag(s)| AgCl(s)| Cl- ((aq,1.0 M)
IV.Hg(l)| Hg2Cl2(s)| Cl- (aq,1.0 M)
V.Hg(s)| Hg2Cl2(s)| Cl- (aq,1.0 M)
A)I and V
B)II and IV
C)I and IV
D)II and III
E)I and III

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
23
Determine the Ksp at 25 °C of AgCl(s).The half-reactions are:
Ag+(aq)+ 1 e- → Ag(s)E° = +0.771 V
AgCl(s)+ 1 e- → Ag(s)+ Cl-(aq) E° = +0.222 V
A)1 × 10-2
B)0.771
C)4 × 1011
D)5 × 10-10
E)4 × 10-11
Ag+(aq)+ 1 e- → Ag(s)E° = +0.771 V
AgCl(s)+ 1 e- → Ag(s)+ Cl-(aq) E° = +0.222 V
A)1 × 10-2
B)0.771
C)4 × 1011
D)5 × 10-10
E)4 × 10-11

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
24
Choose the INCORRECT statement.
A)The work available in a cell = -zFEcell.
B)ΔG ° = -zFE°cell
C)When Ecell < 0,the reaction is spontaneous.
D)When Ecell = 0,the reaction is at equilibrium.
E)Reversing a cell reaction changes the sign of Ecell.
A)The work available in a cell = -zFEcell.
B)ΔG ° = -zFE°cell
C)When Ecell < 0,the reaction is spontaneous.
D)When Ecell = 0,the reaction is at equilibrium.
E)Reversing a cell reaction changes the sign of Ecell.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
25
What is the concentration of Cu2+(aq)in the following cell at 25 °C if the cell voltage is 1.253 V?
Zn(s)∣ Zn2+ (aq,0.125 M) Cu2+(?)∣ Cu(s)
Cu2+(?)∣ Cu(s)
Cu2+(aq)+ 2 e- → Cu(s)E° = 0.340 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.6 M
B)1 × 10-2 M
C)1 × 10-6 M
D)9 × 10-4 M
E)4 × 10-4 M
Zn(s)∣ Zn2+ (aq,0.125 M)
 Cu2+(?)∣ Cu(s)
Cu2+(?)∣ Cu(s)Cu2+(aq)+ 2 e- → Cu(s)E° = 0.340 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.6 M
B)1 × 10-2 M
C)1 × 10-6 M
D)9 × 10-4 M
E)4 × 10-4 M

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
26
If the voltage of an electrochemical cell is negative then the cell reaction is:
A)nonspontaneous
B)slow
C)exothermic
D)spontaneous
E)fast
A)nonspontaneous
B)slow
C)exothermic
D)spontaneous
E)fast

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
27
Determine E°cell for the reaction: 2 Al(aq)+ 3 Zn2+(aq)→ 2 Al3+(aq)+ 3 Zn(s).The half reactions are:
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.913 V
B)-2.439 V
C)2.439 V
D)-1.063 V
E)-0.913 V
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.913 V
B)-2.439 V
C)2.439 V
D)-1.063 V
E)-0.913 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
28
For the reaction: Mg(s)+ AgNO3(aq)→ Ag(s)+ Mg(NO3)2(aq)
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Is the reaction spontaneous and why?
A)No,Mg(s)does not react with Ag+.
B)No,E° is a positive value.
C)No,E° is a negative value.
D)Yes,E° is a positive value.
E)Yes,E° is a negative value.
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Is the reaction spontaneous and why?
A)No,Mg(s)does not react with Ag+.
B)No,E° is a positive value.
C)No,E° is a negative value.
D)Yes,E° is a positive value.
E)Yes,E° is a negative value.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
29
Glass electrodes are used for direct measurement of:
A)pH
B)pKa
C)Ksp
D)Q
E)pKw
A)pH
B)pKa
C)Ksp
D)Q
E)pKw

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
30
The lithium-ion battery is based on:
A)Li+(aq)/Li(s)couple
B)Co3+(aq)/Co2+(aq)couple
C)Co(III)/Co(IV)couple
D)both Li+(aq)/Li(s)and Co3+(aq)/Co2+(aq)
E)both Li+(aq)/Li(s)and Co(III)/Co(IV)
A)Li+(aq)/Li(s)couple
B)Co3+(aq)/Co2+(aq)couple
C)Co(III)/Co(IV)couple
D)both Li+(aq)/Li(s)and Co3+(aq)/Co2+(aq)
E)both Li+(aq)/Li(s)and Co(III)/Co(IV)

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
31
Choose the INCORRECT statement.
A)One mole of hydrogen ions will have a positive charge of one Faraday.
B)One Faraday of charge will release 35.5 g of Cl2(g)in the electrolysis of a chloride solution.
C)One Faraday of charge will plate out two moles of Cu(s)from a solution of CuSO4(aq).
D)One mole of electrons has a charge of l Faraday.
E)One mole of hydrogen gas requires 2 Faradays.
A)One mole of hydrogen ions will have a positive charge of one Faraday.
B)One Faraday of charge will release 35.5 g of Cl2(g)in the electrolysis of a chloride solution.
C)One Faraday of charge will plate out two moles of Cu(s)from a solution of CuSO4(aq).
D)One mole of electrons has a charge of l Faraday.
E)One mole of hydrogen gas requires 2 Faradays.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
32
Choose the INCORRECT statement.
A)A concentration cell consists of half cells with identical electrodes but different ion concentrations.
B)Primary batteries' cell reactions cannot be reversed.
C)Secondary batteries' cell reaction can be reversed.
D)A primary battery is recharged by passing electricity through the battery.
E)Cells can be joined in series to increase the total voltage.
A)A concentration cell consists of half cells with identical electrodes but different ion concentrations.
B)Primary batteries' cell reactions cannot be reversed.
C)Secondary batteries' cell reaction can be reversed.
D)A primary battery is recharged by passing electricity through the battery.
E)Cells can be joined in series to increase the total voltage.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the cell:
Ni(s)∣ Ni2+ (aq,?M)![<strong>Consider the cell: Ni(s)∣ Ni<sup>2+</sup> (aq,?M) Cu<sup>2</sup><sup>+</sup> (aq,0.136 M)∣ Cu(s) Ni<sup>2+</sup>(aq)/Ni(s) E° = -0.257 V Cu<sup>2+</sup>(aq)/Cu(s) E° = 0.340 V The measured potential of the cell is 0.621 V.What is [Ni<sup>2+]</sup> at 25 °C?</strong> A)2 × 10<sup>-2</sup> M B)1 M C)4 × 10<sup>-42</sup> M D)0.05 M E)0.4 M](https://storage.examlex.com/TB5343/11ea7a5e_3e75_98a9_89bd_237cd72380f5_TB5343_11.jpg) Cu2+ (aq,0.136 M)∣ Cu(s)
Cu2+ (aq,0.136 M)∣ Cu(s)
Ni2+(aq)/Ni(s) E° = -0.257 V Cu2+(aq)/Cu(s) E° = 0.340 V
The measured potential of the cell is 0.621 V.What is [Ni2+] at 25 °C?
A)2 × 10-2 M
B)1 M
C)4 × 10-42 M
D)0.05 M
E)0.4 M
Ni(s)∣ Ni2+ (aq,?M)
![<strong>Consider the cell: Ni(s)∣ Ni<sup>2+</sup> (aq,?M) Cu<sup>2</sup><sup>+</sup> (aq,0.136 M)∣ Cu(s) Ni<sup>2+</sup>(aq)/Ni(s) E° = -0.257 V Cu<sup>2+</sup>(aq)/Cu(s) E° = 0.340 V The measured potential of the cell is 0.621 V.What is [Ni<sup>2+]</sup> at 25 °C?</strong> A)2 × 10<sup>-2</sup> M B)1 M C)4 × 10<sup>-42</sup> M D)0.05 M E)0.4 M](https://storage.examlex.com/TB5343/11ea7a5e_3e75_98a9_89bd_237cd72380f5_TB5343_11.jpg) Cu2+ (aq,0.136 M)∣ Cu(s)
Cu2+ (aq,0.136 M)∣ Cu(s)Ni2+(aq)/Ni(s) E° = -0.257 V Cu2+(aq)/Cu(s) E° = 0.340 V
The measured potential of the cell is 0.621 V.What is [Ni2+] at 25 °C?
A)2 × 10-2 M
B)1 M
C)4 × 10-42 M
D)0.05 M
E)0.4 M

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
34
Choose the INCORRECT statement.
A)Corrosion of metals is an oxidation reduction process.
B)Rust is a form of corrosion.
C)Metals can be protected by cathodic protection.
D)Cathodic protection is attaching a more active metal to the protected metal.
E)The active metal is called a sacrificial cathode.
A)Corrosion of metals is an oxidation reduction process.
B)Rust is a form of corrosion.
C)Metals can be protected by cathodic protection.
D)Cathodic protection is attaching a more active metal to the protected metal.
E)The active metal is called a sacrificial cathode.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
35
What is the cell diagram for the spontaneous cell involving the Fe3+(aq)∣ Fe2+(aq)∣ Pt(s)(0.771V)half cell and the Zn2+(aq)∣ Zn(s)(-0.763 V)half cell?
A)Fe2+(aq)∣ Fe3+(aq) Zn2+(aq)∣ Zn(s)
Zn2+(aq)∣ Zn(s)
B)Pt(s)∣ Fe2+(aq),Fe3+(aq) Zn2+(aq)∣ Zn(s)
Zn2+(aq)∣ Zn(s)
C)Zn(s)∣ Zn2+(aq) Fe3+(aq)∣ Fe2+(aq)∣ Pt(s)
Fe3+(aq)∣ Fe2+(aq)∣ Pt(s)
D)Zn(s)∣ Zn2+(aq) Fe3+(aq)∣ Fe2+(aq)
Fe3+(aq)∣ Fe2+(aq)
E)Zn(s)∣ Zn2+(aq) Fe3+(aq)∣ Fe2+(aq)∣ Zn(s)
Fe3+(aq)∣ Fe2+(aq)∣ Zn(s)
A)Fe2+(aq)∣ Fe3+(aq)
 Zn2+(aq)∣ Zn(s)
Zn2+(aq)∣ Zn(s)B)Pt(s)∣ Fe2+(aq),Fe3+(aq)
 Zn2+(aq)∣ Zn(s)
Zn2+(aq)∣ Zn(s)C)Zn(s)∣ Zn2+(aq)
 Fe3+(aq)∣ Fe2+(aq)∣ Pt(s)
Fe3+(aq)∣ Fe2+(aq)∣ Pt(s)D)Zn(s)∣ Zn2+(aq)
 Fe3+(aq)∣ Fe2+(aq)
Fe3+(aq)∣ Fe2+(aq)E)Zn(s)∣ Zn2+(aq)
 Fe3+(aq)∣ Fe2+(aq)∣ Zn(s)
Fe3+(aq)∣ Fe2+(aq)∣ Zn(s)
Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
36
Determine E°cell for the reaction: 2 Ag+(aq)+ Mg(s)→ 2 Ag(s)+ Mg2+(aq).The half reactions are:
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
A)0.756 V
B)-0.756 V
C)3.156 V
D)-1.556 V
E)1.556 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
A)0.756 V
B)-0.756 V
C)3.156 V
D)-1.556 V
E)1.556 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
37
What is the concentration of Al3+(aq)in the following cell at 25 °C if the cell voltage is 1.486 V?
Al(s)∣ Al3+(aq,?) Sn2+(aq,3.21 × 10-4 M)∣ Sn(s)
Sn2+(aq,3.21 × 10-4 M)∣ Sn(s)
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s)E° = -0.137 V
A)2.8 × 10-3 M
B)1.6 × 10-5 M
C)4.5 × 10-5 M
D)2.4 × 10-2 M
E)7.8 × 10-6 M
Al(s)∣ Al3+(aq,?)
 Sn2+(aq,3.21 × 10-4 M)∣ Sn(s)
Sn2+(aq,3.21 × 10-4 M)∣ Sn(s)Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s)E° = -0.137 V
A)2.8 × 10-3 M
B)1.6 × 10-5 M
C)4.5 × 10-5 M
D)2.4 × 10-2 M
E)7.8 × 10-6 M

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the Ksp of lead iodide from the following standard electrode potentials,at 25 °C:
2 e- + PbI2(S)→ Pb(s)+ 2 I-(aq)E° = -0.365 V
2 e- + Pb2+(aq)→ Pb(s)E° = -0.126 V
A)8 × 10-9
B)9 × 10-5
C)2 × 10-17
D)5 × 10-13
E)6 × 10-5
2 e- + PbI2(S)→ Pb(s)+ 2 I-(aq)E° = -0.365 V
2 e- + Pb2+(aq)→ Pb(s)E° = -0.126 V
A)8 × 10-9
B)9 × 10-5
C)2 × 10-17
D)5 × 10-13
E)6 × 10-5

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
39
Determine E°cell for the reaction: Pb2+(aq)+ Zn(s)→ Pb(s)+ Zn2+(aq).The half reactions are:
Pb2+(aq)+ 2 e- → Pb(s)E° = -0.125 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.638 V
B)-0.638 V
C)0.888 V
D)-1.276 V
E)-0.888 V
Pb2+(aq)+ 2 e- → Pb(s)E° = -0.125 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.638 V
B)-0.638 V
C)0.888 V
D)-1.276 V
E)-0.888 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
40
In the cell Pt(s)∣ Fe2+(aq)∣ Fe3+(aq) ![<strong>In the cell Pt(s)∣ Fe<sup>2+</sup>(aq)∣ Fe<sup>3+</sup>(aq) Cu<sup>2+</sup>(aq)∣ Cu(s)which will increase the cell voltage the most?</strong> A)Halve [Cu<sup>2+</sup>]. B)Halve [Fe<sup>2+</sup>]. C)Double [Cu<sup>2+</sup>]. D)Double [Fe<sup>2+</sup>]. E)Cut Cu electrode in half.](https://storage.examlex.com/TB5343/11ea7a5e_3e75_7198_89bd_77df85964174_TB5343_11.jpg) Cu2+(aq)∣ Cu(s)which will increase the cell voltage the most?
Cu2+(aq)∣ Cu(s)which will increase the cell voltage the most?
A)Halve [Cu2+].
B)Halve [Fe2+].
C)Double [Cu2+].
D)Double [Fe2+].
E)Cut Cu electrode in half.
![<strong>In the cell Pt(s)∣ Fe<sup>2+</sup>(aq)∣ Fe<sup>3+</sup>(aq) Cu<sup>2+</sup>(aq)∣ Cu(s)which will increase the cell voltage the most?</strong> A)Halve [Cu<sup>2+</sup>]. B)Halve [Fe<sup>2+</sup>]. C)Double [Cu<sup>2+</sup>]. D)Double [Fe<sup>2+</sup>]. E)Cut Cu electrode in half.](https://storage.examlex.com/TB5343/11ea7a5e_3e75_7198_89bd_77df85964174_TB5343_11.jpg) Cu2+(aq)∣ Cu(s)which will increase the cell voltage the most?
Cu2+(aq)∣ Cu(s)which will increase the cell voltage the most?A)Halve [Cu2+].
B)Halve [Fe2+].
C)Double [Cu2+].
D)Double [Fe2+].
E)Cut Cu electrode in half.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
41
Choose the INCORRECT completion of the following sentence:
"In the electrolysis of water...
A)...a direct current must be used."
B)...oxygen is evolved at the cathode."
C)...electrical energy must be supplied continuously because it is an endothermic reaction."
D)...the volume of hydrogen produced is twice the volume of oxygen produced."
E)....one mole of H2(g)is produced for each two moles of electrons."
"In the electrolysis of water...
A)...a direct current must be used."
B)...oxygen is evolved at the cathode."
C)...electrical energy must be supplied continuously because it is an endothermic reaction."
D)...the volume of hydrogen produced is twice the volume of oxygen produced."
E)....one mole of H2(g)is produced for each two moles of electrons."

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
42
An electrolysis is carried out by passing an electric current through a solution of copper sulfate using inert electrodes.This causes:
A)Cu(s)to plate out on the negative electrode
B)Cu(s)to plate out on the anode
C)oxygen to form on the negative electrode
D)H2(g)to form at the negative electrode
E)sulfur to form on the positive electrode
A)Cu(s)to plate out on the negative electrode
B)Cu(s)to plate out on the anode
C)oxygen to form on the negative electrode
D)H2(g)to form at the negative electrode
E)sulfur to form on the positive electrode

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
43
Determine E°cell at 25 °C for the following reaction:
Pb2+(aq)+ Cu(s)→ Cu2+(aq)+ Pb(s)
The half-reactions are:
Pb2+(aq)+ 2 e- → Pb(s)E° = -0.125 V
Cu2+(aq)+ 2 e- → Cu(s)E° = +0.337 V
A)0.212 V
B)-0.462 V
C)0.462 V
D)-0.212 V
E)0.424 V
Pb2+(aq)+ Cu(s)→ Cu2+(aq)+ Pb(s)
The half-reactions are:
Pb2+(aq)+ 2 e- → Pb(s)E° = -0.125 V
Cu2+(aq)+ 2 e- → Cu(s)E° = +0.337 V
A)0.212 V
B)-0.462 V
C)0.462 V
D)-0.212 V
E)0.424 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
44
Determine E°cell for the reaction: 2 Ag(s)+ Mg2+(aq)→ 2 Ag+(aq)+ Mg.(s)The half reactions are:
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
A)0.756 V
B)-0.756 V
C)3.156 V
D)-3.156 V
E)1.556 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
A)0.756 V
B)-0.756 V
C)3.156 V
D)-3.156 V
E)1.556 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
45
Determine E°cell for the reaction: Pb(s)+ Cu2+(aq)→ Pb2+(aq)+ Cu(s).The half reactions are:
Pb2+(aq)+ 2 e- → Pb(s)E° = -0.125 V
Cu2+(aq)+ 2 e- → Cu(s)E° = 0.340 V
A)0.215 V
B)0.465 V
C)0.930 V
D)-0.215 V
E)-0.465 V
Pb2+(aq)+ 2 e- → Pb(s)E° = -0.125 V
Cu2+(aq)+ 2 e- → Cu(s)E° = 0.340 V
A)0.215 V
B)0.465 V
C)0.930 V
D)-0.215 V
E)-0.465 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
46
Determine E°cell for the reaction: Cl2(g)+ 2 F-(aq)→ 2 Cl-(aq)+ F2(g).The half reactions are:
F2(g)+ 2 e- → 2 F-(aq)E° = +2.866 V
Cl2(g)+ 2 e- → 2 Cl-(aq)E° = 1.358 V
A)3.016 V
B)-4.224 V
C)1.508 V
D)4.224 V
E)-1.508 V
F2(g)+ 2 e- → 2 F-(aq)E° = +2.866 V
Cl2(g)+ 2 e- → 2 Cl-(aq)E° = 1.358 V
A)3.016 V
B)-4.224 V
C)1.508 V
D)4.224 V
E)-1.508 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
47
Determine E°cell for the reaction:
Pb(s)+ Zn2+(aq)→ Pb2+(aq)+ Zn(s).
The half reactions are:
Pb2+(aq)+ 2 e- → Pb(s)E° = -0.125 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.638 V
B)-0.638 V
C)0.888 V
D)-1.276 V
E)-0.888 V
Pb(s)+ Zn2+(aq)→ Pb2+(aq)+ Zn(s).
The half reactions are:
Pb2+(aq)+ 2 e- → Pb(s)E° = -0.125 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.638 V
B)-0.638 V
C)0.888 V
D)-1.276 V
E)-0.888 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
48
Determine E°cell for the reaction: I2(s)+ Cu(s)→ 2 I-(aq)+ Cu2+(aq).The half reactions are:
I2(s)+ 2 e- → 2 I-(aq)E° = +0.535 V
Cu2+(aq)+ 2 e- → Cu(s)E° = 0.340 V
A)0.730 V
B)-0.875 V
C)0.875 V
D)0.195 V
E)-0.195 V
I2(s)+ 2 e- → 2 I-(aq)E° = +0.535 V
Cu2+(aq)+ 2 e- → Cu(s)E° = 0.340 V
A)0.730 V
B)-0.875 V
C)0.875 V
D)0.195 V
E)-0.195 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
49
Write the net redox reaction that occurs in the following galvanic cell.
Ti(s)∣ Ti3+(aq) Se(s)| Se2-(aq)
Se(s)| Se2-(aq)
A)Ti(s)+ Se2-(aq)+ 1 e- → Ti3+(aq)+ Se(s)
B)2 Ti(s)+ 3 Se(s)→ 2 Ti3+(aq)+ 3 Se2-(aq)
C)Ti(s)+ Se(s)→ Ti3+(aq)+ Se2-(aq)+ 1 e-
D)2 Ti3+(aq)+ 3 Se(s)→ 2 Ti(s)+ 3 Se2-(aq)
E)2 Ti3+(aq)+ 3 Se(s)+ 1 e- → 2 Ti(s)+ 3 Se2-(aq)
Ti(s)∣ Ti3+(aq)
 Se(s)| Se2-(aq)
Se(s)| Se2-(aq)A)Ti(s)+ Se2-(aq)+ 1 e- → Ti3+(aq)+ Se(s)
B)2 Ti(s)+ 3 Se(s)→ 2 Ti3+(aq)+ 3 Se2-(aq)
C)Ti(s)+ Se(s)→ Ti3+(aq)+ Se2-(aq)+ 1 e-
D)2 Ti3+(aq)+ 3 Se(s)→ 2 Ti(s)+ 3 Se2-(aq)
E)2 Ti3+(aq)+ 3 Se(s)+ 1 e- → 2 Ti(s)+ 3 Se2-(aq)

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
50
The following half-reactions are used in the zinc-air battery.
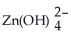 (aq)+ 2 e- → Zn(s)+ 4 OH-(aq)E° = -1.199 V
(aq)+ 2 e- → Zn(s)+ 4 OH-(aq)E° = -1.199 V
O2(g)+ 2 H2O(l)+ 4 e- → 4 OH-(aq)E° = +0.401 V
How much charge is transferred per gram Zn(s)in this voltaic cell?
A)5.90 × 103 C
B)2.95 × 103 C
C)1.47 × 103 C
D)3.39 × 10-4 C
E)1.69 × 10-4 C
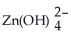 (aq)+ 2 e- → Zn(s)+ 4 OH-(aq)E° = -1.199 V
(aq)+ 2 e- → Zn(s)+ 4 OH-(aq)E° = -1.199 VO2(g)+ 2 H2O(l)+ 4 e- → 4 OH-(aq)E° = +0.401 V
How much charge is transferred per gram Zn(s)in this voltaic cell?
A)5.90 × 103 C
B)2.95 × 103 C
C)1.47 × 103 C
D)3.39 × 10-4 C
E)1.69 × 10-4 C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
51
For the reaction: Mg(s)+ 2 AgNO3(aq)→ 2 Ag(s)+ Mg(NO3)2(aq)
Write a voltaic diagram for the reaction.
A)Ag(s)| Ag(aq) Mg2+(aq)| Mg(s)
Mg2+(aq)| Mg(s)
B)Mg(s)∣ Mg2+ Ag+ (aq)∣ Ag (s)
Ag+ (aq)∣ Ag (s)
C)Mg(s)∣ Mg2+ Ag(s)∣ Ag+ (aq)
Ag(s)∣ Ag+ (aq)
D)Ag(aq)∣ Ag(s) Mg(s)∣ Mg2+ (aq)
Mg(s)∣ Mg2+ (aq)
E)Ag(s)∣ Mg2+ (aq) Ag+ (aq)∣ Mg(s)
Ag+ (aq)∣ Mg(s)
Write a voltaic diagram for the reaction.
A)Ag(s)| Ag(aq)
 Mg2+(aq)| Mg(s)
Mg2+(aq)| Mg(s)B)Mg(s)∣ Mg2+
 Ag+ (aq)∣ Ag (s)
Ag+ (aq)∣ Ag (s)C)Mg(s)∣ Mg2+
 Ag(s)∣ Ag+ (aq)
Ag(s)∣ Ag+ (aq)D)Ag(aq)∣ Ag(s)
 Mg(s)∣ Mg2+ (aq)
Mg(s)∣ Mg2+ (aq)E)Ag(s)∣ Mg2+ (aq)
 Ag+ (aq)∣ Mg(s)
Ag+ (aq)∣ Mg(s)
Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
52
Write the net redox reaction that occurs in the galvanic cell.
Zn(s)∣ Zn+2(aq) Pb+2(aq)∣ Pb(s)
Pb+2(aq)∣ Pb(s)
A)Pb(s)+ Zn(s)→ Pb+2(aq)+ Zn+2(aq)+ 4 e-
B)Pb(s)+ Zn+2(aq)→ Pb+2(aq)+ Zn(s)
C)Pb+2(s)+ Zn+2(s)→ Pb(s)+ Zn(s)+ 4 e-
D)Pb+2(aq)+ Zn(s)→ Zn+2(aq)+ Pb(s)
E)Pb(s)+ Zn(s)+ 4 e- → Pb+2(aq)+ Zn+2(aq)
Zn(s)∣ Zn+2(aq)
 Pb+2(aq)∣ Pb(s)
Pb+2(aq)∣ Pb(s)A)Pb(s)+ Zn(s)→ Pb+2(aq)+ Zn+2(aq)+ 4 e-
B)Pb(s)+ Zn+2(aq)→ Pb+2(aq)+ Zn(s)
C)Pb+2(s)+ Zn+2(s)→ Pb(s)+ Zn(s)+ 4 e-
D)Pb+2(aq)+ Zn(s)→ Zn+2(aq)+ Pb(s)
E)Pb(s)+ Zn(s)+ 4 e- → Pb+2(aq)+ Zn+2(aq)

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
53
How many coulombs would be needed to deposit all of the Ag+(aq)ion from 600 mL of a solution 0.250 M in Ag+(aq)?
A)1.45 × 107 C
B)1.56 × 106 C
C)1.45 × 104 C
D)2.41 × 104 C
E)1.56 × 104 C
A)1.45 × 107 C
B)1.56 × 106 C
C)1.45 × 104 C
D)2.41 × 104 C
E)1.56 × 104 C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
54
Determine E°cell for the reaction: 2 Al3+(aq)+ 3 Zn(s)→ 2 Al + 3 Zn2+(aq).The half reactions are:
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.913 V
B)-2.439 V
C)2.439 V
D)-1.063 V
E)-0.913 V
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)0.913 V
B)-2.439 V
C)2.439 V
D)-1.063 V
E)-0.913 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
55
One mole of electrons has a charge of:
A)96,485 A
B)1.60 × 10-19
C)6.02 × 1023 A
D)96,485 C
E)96,485 F
A)96,485 A
B)1.60 × 10-19
C)6.02 × 1023 A
D)96,485 C
E)96,485 F

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
56
Determine E°cell for the reaction: MnO4-(aq)+ 8 H+(aq)+ 5 Fe2+(aq)→ Mn2+(aq)+ 4 H2O(l)+ 5 Fe3+(aq).The half reactions are:
Fe3+(aq)+ e- → Fe2+(aq)E° = +0.771 V
MnO4-(aq)+ 8 H+(aq)+ 5 e- → Mn2+(aq)+ 4 H2O(l)E° = 1.507 V
A)-2.34 V
B)-2.28 V
C)2.28 V
D)-0.74 V
E)0.74 V
Fe3+(aq)+ e- → Fe2+(aq)E° = +0.771 V
MnO4-(aq)+ 8 H+(aq)+ 5 e- → Mn2+(aq)+ 4 H2O(l)E° = 1.507 V
A)-2.34 V
B)-2.28 V
C)2.28 V
D)-0.74 V
E)0.74 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
57
For the reaction: Mg(s)+ AgNO3(aq)→ Ag(s)+ Mg(NO3)2(aq)
Ag+(aq)+ e- → Ag(s) E° = 0.800 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
For the reaction,determine E° for the cell.
A)1.556 V
B)-3.156 V
C)3.156 V
D)0.800 V
E)2.356 V
Ag+(aq)+ e- → Ag(s) E° = 0.800 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
For the reaction,determine E° for the cell.
A)1.556 V
B)-3.156 V
C)3.156 V
D)0.800 V
E)2.356 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
58
Given the table below predict the numerical value of the standard cell potential for the reaction:
2 Cr(s)+ 3 Cu2+(aq)→ 2 Cr3+(aq)+ 3 Cu(s)
Half Reaction E° (volts)
(1)Cr3+(aq)+ 3 e- → Cr(s)-0.74
(2)Cr3+(aq)+ e- → Cr2+(aq)-0.41
(3)Cr2O72-(aq)+ 14 H+(aq)+ 6 e- → 2 Cr3+(aq)+ 7 H2O(l)1.33
(4)Cu+(aq)+ e- → Cu(s)0.52
(5)Cu2+(aq)+ 2 e- → Cu(s)0.34
(6)Cu2+(aq)+ e- → Cu+(aq)0.16
A)2.50 volts
B)0.417 volts
C)-1.08 volts
D)1.08 volts
E)-0.40 volts
2 Cr(s)+ 3 Cu2+(aq)→ 2 Cr3+(aq)+ 3 Cu(s)
Half Reaction E° (volts)
(1)Cr3+(aq)+ 3 e- → Cr(s)-0.74
(2)Cr3+(aq)+ e- → Cr2+(aq)-0.41
(3)Cr2O72-(aq)+ 14 H+(aq)+ 6 e- → 2 Cr3+(aq)+ 7 H2O(l)1.33
(4)Cu+(aq)+ e- → Cu(s)0.52
(5)Cu2+(aq)+ 2 e- → Cu(s)0.34
(6)Cu2+(aq)+ e- → Cu+(aq)0.16
A)2.50 volts
B)0.417 volts
C)-1.08 volts
D)1.08 volts
E)-0.40 volts

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
59
Will magnesium metal displace Al3+(aq)ion from an aqueous solution?
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
A)No,since E°cell is negative.
B)Yes,since E°cell is negative.
C)No,the reverse reaction is spontaneous.
D)Yes,since E°cell is positive.
E)No,the system is at equilibrium.
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
A)No,since E°cell is negative.
B)Yes,since E°cell is negative.
C)No,the reverse reaction is spontaneous.
D)Yes,since E°cell is positive.
E)No,the system is at equilibrium.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
60
Determine E°cell at 25 °C for the following reaction:
3 Sn2+(aq)+ 2 Al(s)→ 2 Al3+(aq)+ 3 Sn(s)
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s)E° = -0.137 V
A)2.941 V
B)-1.813 V
C)1.813 V
D)-1.539 V
E)1.539 V
3 Sn2+(aq)+ 2 Al(s)→ 2 Al3+(aq)+ 3 Sn(s)
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s)E° = -0.137 V
A)2.941 V
B)-1.813 V
C)1.813 V
D)-1.539 V
E)1.539 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
61
What is E° of the spontaneous cell made from the Zn2+(aq)/Zn(s)(-0.76 V)and Br2(l)/2 Br-(aq)(1.07 V)half cells?
A)-1.83 V
B)0.31 V
C)1.83 V
D)-0.31 V
E)-0.76 V
A)-1.83 V
B)0.31 V
C)1.83 V
D)-0.31 V
E)-0.76 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
62
What is the cell reaction of the spontaneous cell made from the Zn2+(aq)/Zn(s)(-0.76 V)and Br2(l)/2 Br-(aq)(1.07 V)half cells?
A)2Br-(aq)+ Zn2+(aq)→ Zn + Br2(l)
B)Br2(l)+ Zn → Zn2+(aq)+ 2Br-(aq)
C)2Br-(aq)+ Zn → Br2(l)+ Zn2+(aq)
D)Br2(l)+ Zn → Zn2+(aq)+ Br-(aq)
E)Br2(l)+ Zn2+(aq)→ Zn + 2Br-(aq)
A)2Br-(aq)+ Zn2+(aq)→ Zn + Br2(l)
B)Br2(l)+ Zn → Zn2+(aq)+ 2Br-(aq)
C)2Br-(aq)+ Zn → Br2(l)+ Zn2+(aq)
D)Br2(l)+ Zn → Zn2+(aq)+ Br-(aq)
E)Br2(l)+ Zn2+(aq)→ Zn + 2Br-(aq)

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
63
The following galvanic cell has a measured cell potential E°cell = 0.646 V.
Pt(s)∣ Sn4+(aq),Sn2+(aq) Ag+(aq)∣ Ag(s)
Ag+(aq)∣ Ag(s)
Given that the standard reduction potential for the reduction of Ag+(aq)to Ag(s)is +0.800 V,what is the standard reduction potential for the Sn4+/Sn2+ half-reaction?
A)+0.154 V
B)-0.154 V
C)+1.45 V
D)-1.45 V
E)+0.308 V
Pt(s)∣ Sn4+(aq),Sn2+(aq)
 Ag+(aq)∣ Ag(s)
Ag+(aq)∣ Ag(s)Given that the standard reduction potential for the reduction of Ag+(aq)to Ag(s)is +0.800 V,what is the standard reduction potential for the Sn4+/Sn2+ half-reaction?
A)+0.154 V
B)-0.154 V
C)+1.45 V
D)-1.45 V
E)+0.308 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
64
The standard Gibbs energy change for the following voltaic cell is ΔG° = -89.3 kJ at 25 °C.What is E°cell?
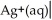 Ag(s)
Ag(s)
A)+0.463 V
B)-0.926 V
C)-0.463 V
D)+0.926 V
E)+0.231 V
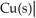
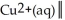
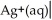 Ag(s)
Ag(s)A)+0.463 V
B)-0.926 V
C)-0.463 V
D)+0.926 V
E)+0.231 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
65
For the cell reaction at 25 °C Cu2+(aq)+ Zn(s)→ Cu(s)+ Zn2+(aq),E°cell = +1.100 V.What is the equilibrium constant for this reaction?
A)3 × 10-19
B)2 × 1037
C)2 × 10-37
D)3 × 1018
E)3 × 10-18
A)3 × 10-19
B)2 × 1037
C)2 × 10-37
D)3 × 1018
E)3 × 10-18

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
66
Calculate Ecell at 25 °C for the following voltaic cell.Ksp for NiCO3(s)is 1.42 × 10-7.
Ni(s)∣ Ni2+(aq)[sat'd NiCO3(s)]![<strong>Calculate E<sub>cell </sub>at 25 °C for the following voltaic cell.K<sub>sp</sub> for NiCO<sub>3</sub>(s)is 1.42 × 10<sup>-7</sup>. Ni(s)∣ Ni<sup>2+</sup>(aq)[sat'd NiCO<sub>3</sub>(s)] Ni<sup>2+</sup>(aq)(0.010M)∣ Ni(s) The half-reaction is: Ni<sup>2+</sup>(aq)+ 2 e<sup>-</sup> → Ni(s)E° = -0.257 V</strong> A)+0.257 V B)-0.257 V C)0.000 V D)+0.0844 V E)+0.0422 V](https://storage.examlex.com/TB5343/11ea7a5e_3e79_9063_89bd_af0b61ba0101_TB5343_11.jpg) Ni2+(aq)(0.010M)∣ Ni(s)
Ni2+(aq)(0.010M)∣ Ni(s)
The half-reaction is: Ni2+(aq)+ 2 e- → Ni(s)E° = -0.257 V
A)+0.257 V
B)-0.257 V
C)0.000 V
D)+0.0844 V
E)+0.0422 V
Ni(s)∣ Ni2+(aq)[sat'd NiCO3(s)]
![<strong>Calculate E<sub>cell </sub>at 25 °C for the following voltaic cell.K<sub>sp</sub> for NiCO<sub>3</sub>(s)is 1.42 × 10<sup>-7</sup>. Ni(s)∣ Ni<sup>2+</sup>(aq)[sat'd NiCO<sub>3</sub>(s)] Ni<sup>2+</sup>(aq)(0.010M)∣ Ni(s) The half-reaction is: Ni<sup>2+</sup>(aq)+ 2 e<sup>-</sup> → Ni(s)E° = -0.257 V</strong> A)+0.257 V B)-0.257 V C)0.000 V D)+0.0844 V E)+0.0422 V](https://storage.examlex.com/TB5343/11ea7a5e_3e79_9063_89bd_af0b61ba0101_TB5343_11.jpg) Ni2+(aq)(0.010M)∣ Ni(s)
Ni2+(aq)(0.010M)∣ Ni(s)The half-reaction is: Ni2+(aq)+ 2 e- → Ni(s)E° = -0.257 V
A)+0.257 V
B)-0.257 V
C)0.000 V
D)+0.0844 V
E)+0.0422 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
67
Find Ecell at 25 °C for the following voltaic cell:
Cu(s)∣ Cu2+(aq)(0.10 M) Cu2+(aq)(0.85 M)∣ Cu(s)
Cu2+(aq)(0.85 M)∣ Cu(s)
The half-reaction is:
Cu2+(aq)+ 2 e- → Cu(s)E° = +0.377 volts
A)+0.0209 V
B)+0.0550 V
C)+0.0275 V
D)-0.0209 V
E)+0.310 V
Cu(s)∣ Cu2+(aq)(0.10 M)
 Cu2+(aq)(0.85 M)∣ Cu(s)
Cu2+(aq)(0.85 M)∣ Cu(s)The half-reaction is:
Cu2+(aq)+ 2 e- → Cu(s)E° = +0.377 volts
A)+0.0209 V
B)+0.0550 V
C)+0.0275 V
D)-0.0209 V
E)+0.310 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
68
The net reaction in a voltaic cell with E°cell = +0.726 V is,
2 Fe3+(aq)+ 3 Zn(s)→ 2 Fe(s)+ 3 Zn2+(aq)
What is ΔG° for this reaction at 25 °C?
A)-210 kJ
B)-140 kJ
C)-700 kJ
D)-463 kJ
E)-420 kJ
2 Fe3+(aq)+ 3 Zn(s)→ 2 Fe(s)+ 3 Zn2+(aq)
What is ΔG° for this reaction at 25 °C?
A)-210 kJ
B)-140 kJ
C)-700 kJ
D)-463 kJ
E)-420 kJ

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
69
What is Keq at 25 °C of the spontaneous cell made from the Zn2+(aq)/Zn(s)(-0.76 V)and Br2(l)/2Br-(aq)(1.07 V)half cells?
A)3 × 1010
B)3 × 10-11
C)8 × 1061
D)2 × 1036
E)5 × 1025
A)3 × 1010
B)3 × 10-11
C)8 × 1061
D)2 × 1036
E)5 × 1025

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
70
Determine Ecell at 25 °C for the following reaction at 25 °C:
Al(s)∣ Al3+(0.435 M) Sn2+(2.12 × 10-3 M)∣ Sn(s)
Sn2+(2.12 × 10-3 M)∣ Sn(s)
3 Sn2+(aq)+ 2 Al(s)→ 2 Al3+(aq)+ 3 Sn(s)
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s)E° = -0.137 V
A)1.227 V
B)1.611 V
C)1.562 V
D)1.487 V
E)1.467 V
Al(s)∣ Al3+(0.435 M)
 Sn2+(2.12 × 10-3 M)∣ Sn(s)
Sn2+(2.12 × 10-3 M)∣ Sn(s)3 Sn2+(aq)+ 2 Al(s)→ 2 Al3+(aq)+ 3 Sn(s)
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s)E° = -0.137 V
A)1.227 V
B)1.611 V
C)1.562 V
D)1.487 V
E)1.467 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
71
Determine Keq at 25 °C for the following reaction:
Pb2+(aq)+ Cu(s)⇌ Cu2+(aq)+ Pb(s)
The half-reactions are:
Pb2+(aq)+ 2 e- ⇌ Pb(s)E° = -0.125 V
Cu2+(aq)+ 2 e- ⇌ Cu(s)E° = +0.337 V
A)2 × 1069
B)3 × 10-16
C)2 × 1016
D)2 × 10-8
E)6 × 107
Pb2+(aq)+ Cu(s)⇌ Cu2+(aq)+ Pb(s)
The half-reactions are:
Pb2+(aq)+ 2 e- ⇌ Pb(s)E° = -0.125 V
Cu2+(aq)+ 2 e- ⇌ Cu(s)E° = +0.337 V
A)2 × 1069
B)3 × 10-16
C)2 × 1016
D)2 × 10-8
E)6 × 107

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
72
Find Ecell for the following voltaic cell at 25 °C:
Ti(s)∣ Ti2+(aq)(0.550M) Sn2+(aq)(0.005 M)∣ Sn(s)
Sn2+(aq)(0.005 M)∣ Sn(s)
The half-reactions are:
Ti2+(aq)+ 2 e- ⇔ Ti(s)E° = -1.630 V
Sn2+(aq)+ 2 e- ⇔ Sn(s)E° = -0.137 V
A)1.372 V
B)1.707 V
C)1.646 V
D)1.433 V
E)-1.646 V
Ti(s)∣ Ti2+(aq)(0.550M)
 Sn2+(aq)(0.005 M)∣ Sn(s)
Sn2+(aq)(0.005 M)∣ Sn(s)The half-reactions are:
Ti2+(aq)+ 2 e- ⇔ Ti(s)E° = -1.630 V
Sn2+(aq)+ 2 e- ⇔ Sn(s)E° = -0.137 V
A)1.372 V
B)1.707 V
C)1.646 V
D)1.433 V
E)-1.646 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
73
What is E° of the spontaneous cell made from Ag+(aq)/Ag(s)(0.80 V)and Cl2(g)/Cl-(aq)(1.36 V)half cells?
A)2.16 V
B)-2.16 V
C)-0.56 V
D)0.56 V
E)1.36 V
A)2.16 V
B)-2.16 V
C)-0.56 V
D)0.56 V
E)1.36 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
74
Using the following reduction half-reactions and their respective standard reduction potentials at 25 °C,draw a diagram of a galvanic cell.
Cr2O72-(aq)+ 14 H+(aq)+ 6e- → 2 Cr3+(aq)+ 7 H2O(l)E° = +1.33 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)Zn2+(aq)∣ Zn(s),H+(aq) Cr2O72-(aq),Cr(s)∣ Cr3+(aq)
Cr2O72-(aq),Cr(s)∣ Cr3+(aq)
B)Zn(s)∣ Zn2+(aq) H+(aq),Cr3+(aq)∣ Cr(s)
H+(aq),Cr3+(aq)∣ Cr(s)
C)Cr(s)∣ Cr3+(aq),H+(aq),Cr2O72-(aq) Zn(S)∣ Zn2+(aq)
Zn(S)∣ Zn2+(aq)
D)Zn(s)∣ Zn2+(aq) Cr2O72-(aq),H+(aq),Cr3+(aq)∣ Pt(s)
Cr2O72-(aq),H+(aq),Cr3+(aq)∣ Pt(s)
E)Pt(s)∣ Cr3+(aq),Cr2O72-(aq),H+(aq) Zn2+(aq)∣ Zn(s)
Zn2+(aq)∣ Zn(s)
Cr2O72-(aq)+ 14 H+(aq)+ 6e- → 2 Cr3+(aq)+ 7 H2O(l)E° = +1.33 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
A)Zn2+(aq)∣ Zn(s),H+(aq)
 Cr2O72-(aq),Cr(s)∣ Cr3+(aq)
Cr2O72-(aq),Cr(s)∣ Cr3+(aq)B)Zn(s)∣ Zn2+(aq)
 H+(aq),Cr3+(aq)∣ Cr(s)
H+(aq),Cr3+(aq)∣ Cr(s)C)Cr(s)∣ Cr3+(aq),H+(aq),Cr2O72-(aq)
 Zn(S)∣ Zn2+(aq)
Zn(S)∣ Zn2+(aq)D)Zn(s)∣ Zn2+(aq)
 Cr2O72-(aq),H+(aq),Cr3+(aq)∣ Pt(s)
Cr2O72-(aq),H+(aq),Cr3+(aq)∣ Pt(s)E)Pt(s)∣ Cr3+(aq),Cr2O72-(aq),H+(aq)
 Zn2+(aq)∣ Zn(s)
Zn2+(aq)∣ Zn(s)
Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
75
For the reaction: Mg(s)+ AgNO3(aq)→ Ag(s)+ Mg(NO3)2(aq)
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Determine ΔG°.
A)-300.3 kJ/mol
B)304.5 kJ/mol
C)609.0 kJ/mol
D)-304.5 kJ/mol
E)-609.0 kJ/mol
Ag+(aq)+ e- → Ag(s)E° = 0.800 V
Mg2+(aq)+ 2 e- → Mg(s)E° = -2.356 V
Determine ΔG°.
A)-300.3 kJ/mol
B)304.5 kJ/mol
C)609.0 kJ/mol
D)-304.5 kJ/mol
E)-609.0 kJ/mol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
76
What is ΔG° at 25 °C for the reaction (not balanced): C3H8(g)+ O2(g)→ CO2(g)+ H2O(l)if the standard cell potential is 1.092 V?
A)-105 kJ
B)-211 kJ
C)105 kJ
D)-2107 kJ
E)211 kJ
A)-105 kJ
B)-211 kJ
C)105 kJ
D)-2107 kJ
E)211 kJ

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
77
What is the cell diagram for the spontaneous cell involving the Cl2(g)∣ Cl-(aq)(1.358 V)and the Sn2+(aq)∣ Sn(s)(-0.137V)half cells?
A)Pt(s)∣ Cl2(g)∣ Cl-(aq) Sn2+(aq)∣ Sn(s)
Sn2+(aq)∣ Sn(s)
B)Sn(s)∣ Sn2+(aq) Cl-(aq)∣ Cl2(g)∣ Pt(s)
Cl-(aq)∣ Cl2(g)∣ Pt(s)
C)Cl2(g)∣ Cl-(aq) Sn2+(aq)∣ Sn(s)
Sn2+(aq)∣ Sn(s)
D)Sn(s) Sn2+(aq)
Sn2+(aq)  Cl-(aq)∣ Cl2(g)
Cl-(aq)∣ Cl2(g)
E)Sn(s)∣ Cl-(aq) Sn2+(aq)∣ Sn(s)
Sn2+(aq)∣ Sn(s)
A)Pt(s)∣ Cl2(g)∣ Cl-(aq)
 Sn2+(aq)∣ Sn(s)
Sn2+(aq)∣ Sn(s)B)Sn(s)∣ Sn2+(aq)
 Cl-(aq)∣ Cl2(g)∣ Pt(s)
Cl-(aq)∣ Cl2(g)∣ Pt(s)C)Cl2(g)∣ Cl-(aq)
 Sn2+(aq)∣ Sn(s)
Sn2+(aq)∣ Sn(s)D)Sn(s)
 Sn2+(aq)
Sn2+(aq)  Cl-(aq)∣ Cl2(g)
Cl-(aq)∣ Cl2(g)E)Sn(s)∣ Cl-(aq)
 Sn2+(aq)∣ Sn(s)
Sn2+(aq)∣ Sn(s)
Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
78
What is Keq at 25 °C of the spontaneous cell made from Ag+(aq)/Ag(s)(0.80 V)and Cl2(g)/Cl-(aq)(1.36 V)half cells?
A)9 × 10-74
B)1 × 10-19
C)1 × 1073
D)9 × 1018
E)1 × 1027
A)9 × 10-74
B)1 × 10-19
C)1 × 1073
D)9 × 1018
E)1 × 1027

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
79
What is the standard cell potential if the Gibbs energy is -2108 kJ in the reaction:
C3H8(g)+ O2(g)→ CO2(g)+ H2O(l)(not balanced)?
A)-10.92 V
B)10.92 V
C)21.85 V
D)-1.092 V
E)1.092 V
C3H8(g)+ O2(g)→ CO2(g)+ H2O(l)(not balanced)?
A)-10.92 V
B)10.92 V
C)21.85 V
D)-1.092 V
E)1.092 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
80
What is Ecell at 25 °C of the spontaneous cell made from Ag+(aq)/Ag(s)(0.80 V)and Cl2(g)/Cl-(aq)(1.36 V)half cells if [Ag+] = 1 × 10-2 M and [Cl-] = 1 × 10-4 M with P(Cl2)= 1.1 atm?
A)0.65 V
B)0.38 V
C)0.74 V
D)0.92 V
E)0.20 V
A)0.65 V
B)0.38 V
C)0.74 V
D)0.92 V
E)0.20 V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck


