Exam 19: Electrochemistry
Exam 1: Matter: Its Properties and Measurement136 Questions
Exam 2: Atoms and the Atomic Theory119 Questions
Exam 3: Chemical Compounds152 Questions
Exam 4: Chemical Reactions170 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions124 Questions
Exam 6: Gases113 Questions
Exam 7: Thermochemistry125 Questions
Exam 8: Electrons in Atoms123 Questions
Exam 9: The Periodic Table and Some Atomic Properties93 Questions
Exam 10: Chemical Bonding I: Basic Concepts107 Questions
Exam 11: Chemical Bonding Ii: Valence Bond and Molecular Orbital Theories104 Questions
Exam 12: Intermolecular Forces: Liquids and Solids121 Questions
Exam 13: Spontaneous Change: Entropy and Gibbs Energy123 Questions
Exam 14: Solutions and Their Physical Properties132 Questions
Exam 15: Principles of Chemical Equilibrium118 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Additional Aspects of Acidbase Equilibria130 Questions
Exam 18: Solubility and Complex-Ion Equilibria104 Questions
Exam 19: Electrochemistry127 Questions
Exam 20: Chemical Kinetics124 Questions
Exam 21: Chemistry of the Main-Group Elements I: Groups 1,2,13,and 14116 Questions
Exam 22: Chemistry of the Main-Group Elements Ii: Groups 18,17,16,15,and Hydrogen100 Questions
Exam 23: The Transition Elements108 Questions
Exam 24: Complex Ions and Coordination Compounds104 Questions
Exam 25: Nuclear Chemistry116 Questions
Exam 26: Structures of Organic Compounds99 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State104 Questions
Select questions type
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten 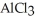 (l)with an electrical current of 12.0 A?
(l)with an electrical current of 12.0 A?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
E
Using the following reduction half-reactions and their respective standard reduction potentials at 25 °C,draw a diagram of a galvanic cell.
Cr2O72-(aq)+ 14 H+(aq)+ 6e- → 2 Cr3+(aq)+ 7 H2O(l)E° = +1.33 V
Zn2+(aq)+ 2 e- → Zn(s)E° = -0.763 V
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
D
Determine E°cell for the reaction: I2(s)+ Cu(s)→ 2 I-(aq)+ Cu2+(aq).The half reactions are:
I2(s)+ 2 e- → 2 I-(aq)E° = +0.535 V
Cu2+(aq)+ 2 e- → Cu(s)E° = 0.340 V
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
D
What is the reduction half-reaction for the following overall galvanic cell reaction?
Co2+(aq)+ 2Ag(s)→ Co(s)+ 2 Ag+(aq)
(Multiple Choice)
4.8/5  (37)
(37)
How long must a constant current of 50.0 A be passed through an electrolytic cell containing aqueous Cu2+ ions to produce 5.00 moles of copper metal?
(Multiple Choice)
5.0/5  (34)
(34)
What is E° of the spontaneous cell made from Ag+(aq)/Ag(s)(0.80 V)and Cl2(g)/Cl-(aq)(1.36 V)half cells?
(Multiple Choice)
4.8/5  (35)
(35)
Write the net redox reaction that occurs in the galvanic cell.
Zn(s)∣ Zn+2(aq)  Pb+2(aq)∣ Pb(s)
Pb+2(aq)∣ Pb(s)
(Multiple Choice)
4.9/5  (41)
(41)
What is the cell reaction of the spontaneous cell made from the Zn2+(aq)/Zn(s)(-0.76 V)and Br2(l)/2 Br-(aq)(1.07 V)half cells?
(Multiple Choice)
4.8/5  (37)
(37)
3.53 L of 0.362 M CuCl2(aq)solution are electrolyzed for 55.6 minutes with a current of 14.0-amperes.What is [CuCl2] when the electrolysis ends?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following are commonly used as alternate standard electrodes:
I.Ag(s)| AgCl(s)| Cl- ((aq,1.0 M)
II.Hg(l)| HgCl2(s)| Cl- (aq,1.0 M)
III.Ag(s)| AgCl(s)| Cl- ((aq,1.0 M)
IV.Hg(l)| Hg2Cl2(s)| Cl- (aq,1.0 M)
V.Hg(s)| Hg2Cl2(s)| Cl- (aq,1.0 M)
(Multiple Choice)
4.7/5  (24)
(24)
A galvanic cell consists of a Ni2+(aq)/Ni(s)half-cell and a standard hydrogen electrode.If the Ni2+/Ni half-cell standard cell functions as the anode,and the standard cell potential is 0.26 V,what is the standard reduction potential for the Ni2+(aq)/Ni(s)half-cell?
(Multiple Choice)
4.7/5  (28)
(28)
For a galvanic cell that uses the following two half-reactions,
Cr2O72-(aq)+ 14 H+(aq)+ 6 e- → 2 Cr3+(aq)+ 7 H2O(l)
Pb(s)→ Pb2+(aq)+ 2 e-
How many moles of Pb(s)are oxidized by three moles of Cr2O72-(aq)?
(Multiple Choice)
4.8/5  (44)
(44)
What is the cell potential for the following cell at 25 °C?
Al(s)∣ Al3+(0.00212 M)  Sn2+(0.435 M)∣ Sn(s)
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s E° = -0.137 V
Sn2+(0.435 M)∣ Sn(s)
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s E° = -0.137 V
(Multiple Choice)
4.9/5  (39)
(39)
Using the following standard reduction potentials,
Fe3+(aq)+ e- → Fe2+(aq)E° = +0.77 V
Ni2+(aq)+ 2 e- → Ni(s)E° = -0.23 V
Calculate the standard cell potential for the galvanic cell reaction given below and determine whether or not this reaction is spontaneous under standard conditions.
Ni2+(aq)+ 2 Fe2+(aq)→ 2 Fe3+(aq)+ Ni(s)
(Multiple Choice)
4.9/5  (39)
(39)
What is the concentration of Al3+(aq)in the following cell at 25 °C if the cell voltage is 1.486 V?
Al(s)∣ Al3+(aq,?)  Sn2+(aq,3.21 × 10-4 M)∣ Sn(s)
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s)E° = -0.137 V
Sn2+(aq,3.21 × 10-4 M)∣ Sn(s)
Al3+(aq)+ 3 e- → Al(s)E° = -1.676 V
Sn2+(aq)+ 2 e- → Sn(s)E° = -0.137 V
(Multiple Choice)
4.8/5  (29)
(29)
How many coulombs would be needed to deposit all of the Ag+(aq)ion from 600 mL of a solution 0.250 M in Ag+(aq)?
(Multiple Choice)
4.8/5  (33)
(33)
For the reaction: Mg(s)+ 2 AgNO3(aq)→ 2 Ag(s)+ Mg(NO3)2(aq)
Write a voltaic diagram for the reaction.
(Multiple Choice)
4.9/5  (37)
(37)
What is the balanced chemical equation for the galvanic cell reaction expressed using shorthand notation below?
Al(s)∣ Al3+(aq)∣∣ Fe2+(aq)∣ Fe(s)
(Multiple Choice)
4.9/5  (30)
(30)
Showing 1 - 20 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)